| Citation: | Yuliang Yuan, Zhilong Yang, Wenchuan Lai, Jiawei Zhang, Xuli Chen, Hongwen Huang. Rational design and preparation of core-shell nanomaterials to boost their catalytic performance[J]. Energy Lab, 2023, 1(2): 220021. doi: 10.54227/elab.20220021 |
Rational design and preparation of core-shell nanomaterials to boost their catalytic performance
-
Abstract
From the morphological point of view, catalysts can be classified into zero-dimensional (nanoparticle or quantum dot), one-dimensional (nanowire), two-dimensional (nanosheet), three-dimensional, and a combination of them. Among the varieties of morphology, core-shell structural catalysts with three-dimensional configuration stand out due to their unique construction and rich forms of interaction between the core and the shell, as well as their abundant ways of interaction with the catalytic intermediates. Constructing high-performance core-shell structural catalysts relies on the comprehensive understanding of the catalytic process and precise control over the catalyst structure. Here in this review, we attempt to sort out common synthetic methods for catalysts with core-shell structures from basic techniques to complex multiple processes. We will analyze how the core-shell configuration affects the catalytic performance from the microscopic to mesoscopic scales. We would resolve the structure-property relationship from the aspects of activity, selectivity, and durability, respectively. Finally, we would end this review with perspectives on the future development of core-shell catalysts.
-
Keywords:
- activity /
- catalyst /
- durability /
- selectivity /
- structure-performance relationship /
- structure configuration
-
-
References
1. R. F. Wang, H. Wang, F. Luo, S. J. Liao, Electrochem. Energy Rev., 2018, 1, 324 2. B. Liu, S. J. Liao, Z. X. Liang, Prog. Chem., 2011, 23, 852. 3. X. Wang, B. He, Z. Hu, Z. Zeng, S. Han, Sci. Technol. Adv. Mater., 2014, 15, 043502 4. S. T. Hunt, Y. Roman-Leshkov, Acc. Chem. Res., 2018, 51, 1054 5. S. Das, J. Perez-Ramirez, J. L. Gong, N. Dewangan, K. Hidajat, B. C. Gates, S. Kawi, Chem. Soc. Rev., 2020, 49, 2937 6. N. V. Long, Y. Yang, C. M. Thi, N. V. Minh, Y. Q. Cao, M. Nogami, Nano Energy, 2013, 2, 636 7. J. J. Ge, Z. J. Li, X. Hong, Y. D. Li, Chem. -Eur. J., 2019, 25, 5113 8. B. Hammer, J. K. Norskov, Nature, 1995, 376, 238 9. J. Greeley, I. E. Stephens, A. S. Bondarenko, T. P. Johansson, H. A. Hansen, T. F. Jaramillo, J. Rossmeisl, I. Chorkendorff, J. K. Norskov, Nat. Chem., 2009, 1, 552 10. S. H. Joo, J. Y. Park, C. K. Tsung, Y. Yamada, P. D. Yang, G. A. Somorjai, Nat. Mater., 2009, 8, 126 11. D. Gohl, A. Garg, P. Paciok, K. J. J. Mayrhofer, M. Heggen, Y. Shao-Horn, R. E. Dunin-Borkowski, Y. Roman-Leshkov, M. Ledendecker, Nat. Mater., 2020, 19, 287 12. S. Hu, W. X. Li, Science, 2021, 374, 1360 13. T. Tang, W. J. Jiang, X. Z. Liu, J. Deng, S. Niu, B. Wang, S. F. Jin, Q. Zhang, L. Gu, J. S. Hu, L. J. Wan, J. Am. Chem. Soc., 2020, 142, 7116 14. L.-P. Yuan, T. Tang, J.-S. Hu, L.-J. Wan, Acc. Mater. Res., 2021, 2, 907 15. E. Gioria, L. Duarte-Correa, N. Bashiri, W. Hetaba, R. Schomaecker, A. Thomas, Nanoscale Adv., 2021, 3, 3454 16. Z. J. Wang, J. Qi, N. L. Yang, R. B. Yu, D. Wang, Mater. Chem. Front., 2021, 5, 1126 17. M. Zhao, K. Deng, L. He, Y. Liu, G. Li, H. Zhao, Z. Tang, J. Am. Chem. Soc., 2014, 136, 1738 18. S. Y. Xue, G. Y. Chen, F. Li, Y. H. Zhao, Q. W. Zeng, J. H. Peng, F. L. Shi, W. C. Zhang, Y. Z. Wang, J. B. Wu, R. C. Che, Small, 2021, 17, 2100559 19. J. H. Hodak, A. Henglein, M. Giersig, G. V. Hartland, J. Phys. Chem. B, 2000, 104, 11708 20. R. Harpeness, A. Gedanken, Langmuir, 2004, 20, 3431 21. N. Ghows, M. H. Entezari, Ultrason. Sonochem., 2011, 18, 629 22. R. Ghosh Chaudhuri, S. Paria, Chem. Rev., 2012, 112, 2373 23. W. Stber, A. Fink, E. Bohn, J. Colloid Interface Sci., 1968, 26, 62 24. N. Avci, P. F. Smet, H. Poelman, N. Van de Velde, K. De Buysser, I. Van Driessche, D. Poelman, J. Sol-Gel Sci. Technol., 2009, 52, 424 25. B. Dong, C. R. Li, X. J. Wang, J. Sol-Gel Sci. Technol., 2007, 44, 161 26. H. Xiao, Z. Ai, L. Zhang, J. Phys. Chem. C, 2009, 113, 16625 27. M. Alifanti, B. Baps, N. Blangenois, J. Naud, P. Grange, B. Delmon, Chem. Mater., 2003, 15, 395 28. Y. F. Lim, C. S. Chua, C. J. Lee, D. Chi, Phys. Chem. Chem. Phys., 2014, 16, 25928 29. L. M. Liz-Marzán, M. Giersig, P. Mulvaney, Langmuir, 1996, 12, 4329 30. G. Büchel, K. K. Unger, A. Matsumoto, K. Tsutsumi, Adv. Mater., 1998, 10, 1036 31. Y. Deng, D. Qi, C. Deng, X. Zhang, D. Zhao, J. Am. Chem. Soc., 2008, 130, 28 32. Q. He, Z. Zhang, J. Xiong, Y. Xiong, H. Xiao, Opt. Mater., 2008, 31, 380 33. W. Li, J. Yang, Z. Wu, J. Wang, B. Li, S. Feng, Y. Deng, F. Zhang, D. Zhao, J. Am. Chem. Soc., 2012, 134, 11864 34. K. Tedsree, T. Li, S. Jones, C. W. Chan, K. M. Yu, P. A. Bagot, E. A. Marquis, G. D. Smith, S. C. Tsang, Nat. Nanotechnol., 2011, 6, 302 35. X. Wang, S. I. Choi, L. T. Roling, M. Luo, C. Ma, L. Zhang, M. Chi, J. Liu, Z. Xie, J. A. Herron, M. Mavrikakis, Y. Xia, Nat. Commun., 2015, 6, 7594 36. D. Liu, S. Q. Lu, Y. R. Xue, Z. Guan, J. J. Fang, W. Zhu, Z. B. Zhuang, Nano Energy, 2019, 59, 26 37. K. A. Kuttiyiel, Y. Choi, K. Sasaki, D. Su, S. M. Hwang, S. D. Yim, T. H. Yang, G. G. Park, R. R. Adzic, Nano Energy, 2016, 29, 261 38. S. J. Seo, H. K. Chung, J. B. Yoo, H. Chae, S. W. Seo, S. M. Cho, J. Vac. Sci. Technol. A, 2014, 32, 01A1298 39. C. Y. He, X. M. Bu, S. W. Yang, P. He, G. Q. Ding, X. M. Xie, Appl. Surf. Sci., 2018, 436, 373 40. D. Wang, H. L. Xin, R. Hovden, H. Wang, Y. Yu, D. A. Muller, F. J. DiSalvo, H. D. Abruna, Nat. Mater., 2013, 12, 81 41. K. J. Mayrhofer, V. Juhart, K. Hartl, M. Hanzlik, M. Arenz, Angew. Chem. Int. Ed., 2009, 48, 3529 42. M. Oezaslan, F. Hasché, P. Strasser, J. Phys. Chem. Lett., 2013, 4, 3273 43. X. Tian, X. Zhao, Y. Q. Su, L. Wang, H. Wang, D. Dang, B. Chi, H. Liu, E. J. M. Hensen, X. W. D. Lou, B. Y. Xia, Science, 2019, 366, 850 44. P. Strasser, S. Koh, T. Anniyev, J. Greeley, K. More, C. Yu, Z. Liu, S. Kaya, D. Nordlund, H. Ogasawara, M. F. Toney, A. Nilsson, Nat. Chem., 2010, 2, 454 45. D. Li, X. Zhang, M. Ramzan, K. Gu, Y. Chen, J. Zhang, B. Zou, H. Zhong, Chem. Mater., 2020, 32, 6650 46. X. Li, M. Su, Y. C. Wang, M. Xu, M. Tong, S. J. Haigh, J. Zhang, Inorg. Chem., 2022, 61, 3989 47. N. Nagelj, A. Brumberg, S. Peifer, R. D. Schaller, J. H. Olshansky, J. Phys. Chem. Lett., 2022, 13, 3209 48. Y. Yan, Z. Zhou, X. Zhao, J. Zhou, J. Colloid Interface Sci., 2014, 435, 91 49. J. Tian, T. Yan, Z. Qiao, L. Wang, W. Li, J. You, B. Huang, Appl. Catal. B: Environ., 2017, 209, 566 50. G. A. Kamat, C. Yan, W. T. Osowiecki, I. A. Moreno-Hernandez, M. Ledendecker, A. P. Alivisatos, J. Phys. Chem. Lett., 2020, 11, 5318 51. D. Liu, W. Li, X. Feng, Y. Zhang, Chem. Sci., 2015, 6, 7015 52. N. Teo, C. Jin, A. Kulkarni, S. C. Jana, J. Colloid Interface Sci., 2020, 561, 772 53. D. Liu, J. Yan, K. Wang, Y. Wang, G. Luo, Nano Res., 2021, 15, 1199 54. A. J. Medford, A. Vojvodic, J. S. Hummelshj, J. Voss, F. Abild-Pedersen, F. Studt, T. Bligaard, A. Nilsson, J. K. Nrskov, J. Catal., 2015, 328, 36 55. A. L. Strickler, A. Jackson, T. F. Jaramillo, ACS Energy Lett., 2017, 2, 244 56. J. E. S. van der Hoeven, J. Jelic, L. A. Olthof, G. Totarella, R. J. A. van Dijk-Moes, J. M. Krafft, C. Louis, F. Studt, A. van Blaaderen, P. E. de Jongh, Nat. Mater., 2021, 20, 1216 57. H. B. Zhang, Z. J. Ma, J. J. Duan, H. M. Liu, G. G. Liu, T. Wang, K. Chang, M. Li, L. Shi, X. G. Meng, K. C. Wu, J. H. Ye, ACS Nano, 2016, 10, 684 58. H. Xie, S. Q. Chen, J. S. Liang, T. Y. Wang, Z. F. Hou, H. L. Wang, G. L. Chai, Q. Li, Adv. Funct. Mater., 2021, 31, 2100883 59. Y. Xing, X. Kong, X. Guo, Y. Liu, Q. Li, Y. Zhang, Y. Sheng, X. Yang, Z. Geng, J. Zeng, Adv. Sci., 2020, 7, 1902989 60. D. Yu, L. Gao, T. Sun, J. Guo, Y. Yuan, J. Zhang, M. Li, X. Li, M. Liu, C. Ma, Q. Liu, A. Pan, J. Yang, H. Huang, Nano Lett., 2021, 21, 1003 61. S. Aguado, S. El-Jamal, F. Meunier, J. Canivet, D. Farrusseng, Chem. Commun., 2016, 52, 7161 62. Y. Long, S. Song, J. Li, L. Wu, Q. Wang, Y. Liu, R. Jin, H. Zhang, ACS Catal., 2018, 8, 8506 63. Z. Shang, X. Liang, Nano Lett., 2017, 17, 104 64. M. Cai, Y. Li, Q. Liu, Z. Xue, H. Wang, Y. Fan, K. Zhu, Z. Ke, C.-Y. Su, G. Li, Adv. Sci., 2019, 6, 1802365 65. Y. Xu, X. Li, J. Gao, J. Wang, G. Ma, X. Wen, Y. Yang, Y. Li, M. Ding, Science, 2021, 371, 610 66. X. Y. Zhang, W. J. Li, X. F. Wu, Y. W. Liu, J. Chen, M. Zhu, H. Y. Yuan, S. Dai, H. F. Wang, Z. Jiang, P. F. Liu, H. G. Yang, Energy Environ. Sci., 2022, 15, 234 67. S. S. Zhang, S. L. Zhao, D. X. Qu, X. J. Liu, Y. P. Wu, Y. H. Chen, W. Huang, Small, 2021, 17, 2102293 68. Y. Zhao, H. Zhou, X. Zhu, Y. Qu, C. Xiong, Z. Xue, Q. Zhang, X. Liu, F. Zhou, X. Mou, W. Wang, M. Chen, Y. Xiong, X. Lin, Y. Lin, W. Chen, H.-J. Wang, Z. Jiang, L. Zheng, T. Yao, J. Dong, S. Wei, W. Huang, L. Gu, J. Luo, Y. Li, Y. Wu, Nat. Catal., 2021, 4, 134 69. L. Adijanto, D. A. Bennett, C. Chen, A. S. Yu, M. Cargnello, P. Fornasiero, R. J. Gorte, J. M. Vohs, Nano Lett., 2013, 13, 2252 70. M. Karuppannan, Y. Kim, S. Gok, E. Lee, J. Y. Hwang, J. H. Jang, Y. H. Cho, T. Lim, Y. E. Sung, O. J. Kwon, Energy Environ. Sci., 2019, 12, 2820 71. T. O. He, W. C. Wang, X. L. Yang, F. L. Shi, Z. Y. Ye, Y. Z. Zheng, F. Li, J. B. Wu, Y. D. Yin, M. S. Jin, ACS Nano, 2021, 15, 7348 72. L. Gloag, T. M. Benedetti, S. Cheong, R. F. Webster, C. E. Marjo, J. J. Gooding, R. D. Tilley, Nanoscale, 2018, 10, 15173 -
Rights and permissions
This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.
Information
Article Metrics
-
Figure 1.
Graphical illustration of the common methods for the synthesis of core-shell nanomaterials.
-
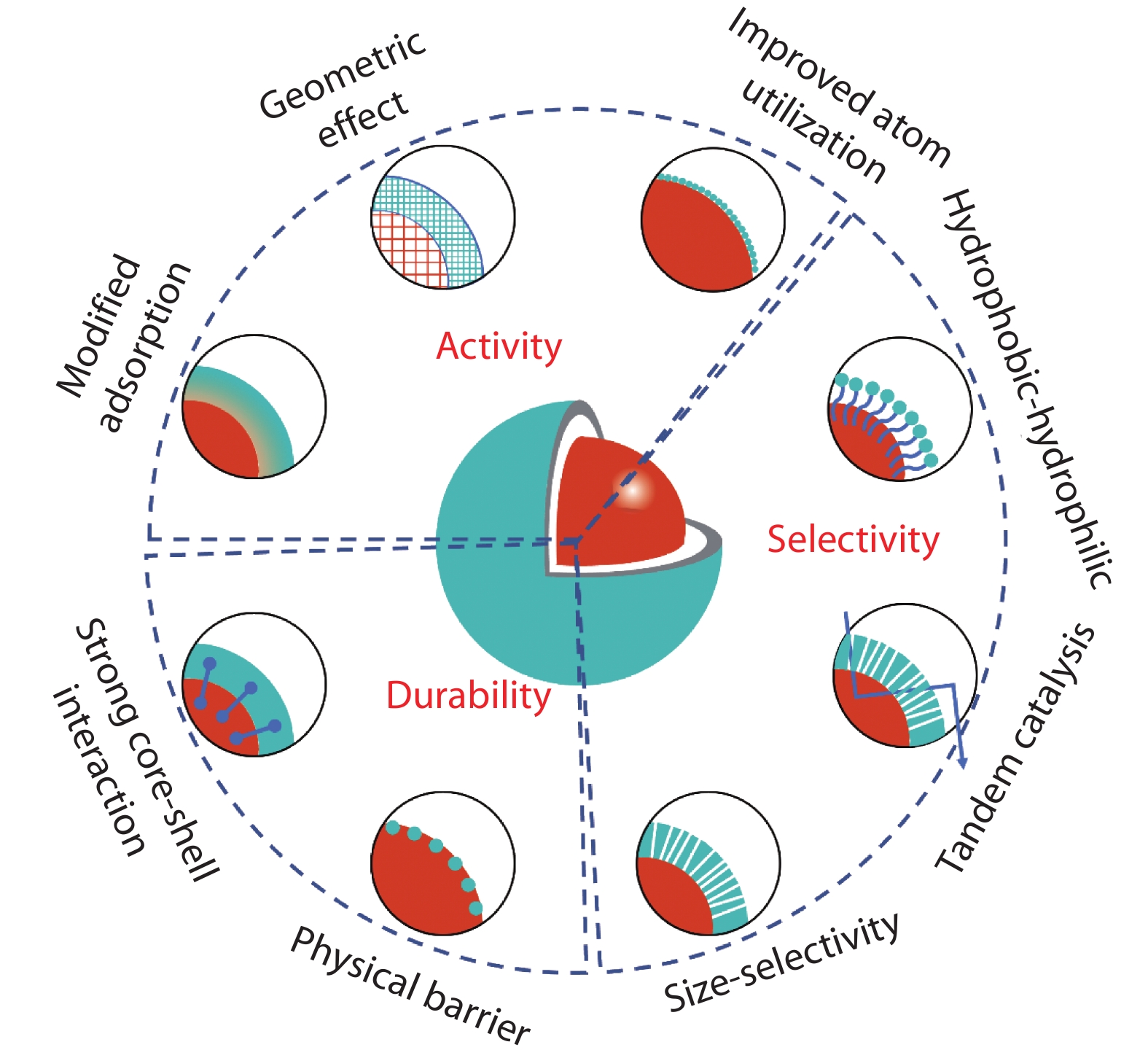
Figure 2.
Schematic illustration shows the configuration of core-shell structure on the effects of catalysis.
-
Figure 3.
a Atomic model and TEM image of Ir@Pt core-shell nanoparticle. b Mass activity and specific activity of Pt/C and Ir@Pt at 0.9 VRHE before and after
10000 stability cycles.[55] Copyright 2016, American Chemical Society. c TEM image and EDX maps show the structure and composition of Au@Pd core-shell nanorod. d TOF and selectivity at 98% butadiene conversion as functions of the Pd fraction.[56] Copyright 2021, Springer Nature Limited. -
Figure 4.
a TEM image of a representative CuPd@Pd nanoparticle. b HER polarization curves of Cu/C, Cu@Pd/C, Pd/C, and Pt/C catalysts in 0.5 M H2SO4 electrolyte. c ORR polarization curves of Cu@Pd/C, Pd/C, and Pt/C catalysts in 0.1 M HClO4 electrolyte.[58] Copyright 2021, Wiley-VCH GmbH. d TEM image of Bi@Sn core-shell nanoparticle. e HAADF-STEM image of Bi@Sn nanoparticle. f Faradaic efficiency of HCOOH on Bi@Sn nanoparticles and Sn nanoparticles.[59] Copyright 2020, The Authors. g Schematic illustration shows the geometric interaction between ordered AuCu3 core and fct Au shell. h Faradaic efficiency of HCOOH on o-AuCu3@fct Au nanoparticle and fcc Au nanoparticle.[60] Copyright 2021, American Chemical Society.
-
Figure 5.
a Schematic illustration for the preparation of the Pt/Al2O3@SIM-1 sphere. Ethylene b and Toluene c hydrogenation on Pt/Al2O3 and Pt/Al2O3@SIM-1 catalysts.[61] Copyright 2016, The Royal Society of Chemistry. d Schematic illustration of the wettable Cu, hydrophobic Cu, and the atomic model of the interface between Cu and C coating. e From left to right: *CO desorption to form CO(g), *CO protonation to form *CHO or *COH, and C-C coupling to form *OCCO on Cu. f Free energies for the three competing reactions under different H2O quantities.[66] Copyright 2022, The Royal Society of Chemistry.
-
Figure 6.
a Schematic illustration shows the integration of two catalysts into one particle by constructing the core-shell structure. b TEM image of Pd@IRMOF-3 nanocomposites. c Synthetic route for Pd@IRMOF-3 hybrids. d cascade reactions involving Knoevenagel condensation of A and Malononitrile via the IRMOF-3 shell and subsequent selective hydrogenation of intermediate product B to C via the Pd nanoparticle core.[17] Copyright 2014, American Chemical Society. e-f Elemental mappings images for Cu@Ag, red for copper and green for silver. g Schematic illustration of tandem catalysis for CO2 reduction to C2 over Cu@Ag core-shell nanoparticle.[67] Copyright 2021, Wiley-VCH GmbH.
-
Figure 7.
a Schematic illustration of the synthesis of Pt@mSiO2 nanoparticles. Thermal stability of Pt@mSiO2 nanoparticles after heat treatment at b 350 °C, c 550 °C, and d 750 °C.[10] Copyright 2009, Macmillan Publishers Limited. e Pd@CeO2 loaded on alkyl-siloxane functionalized YSZ (100) after heat treatment in air at
1373 K. f Pd@CeO2 loaded on pristine YSZ (100) after heat treatment in air at1373 K.[69] Copyright 2013, American Chemical Society. g TEM image of Pt@C/CNF. h High magnification TEM image of Pt@C/CNF. i Mass activity of Pt/C and Pt@C/CNF according to the number of accelerated stability test cycles.[70] Copyright 2019, The Royal Society of Chemistry. -
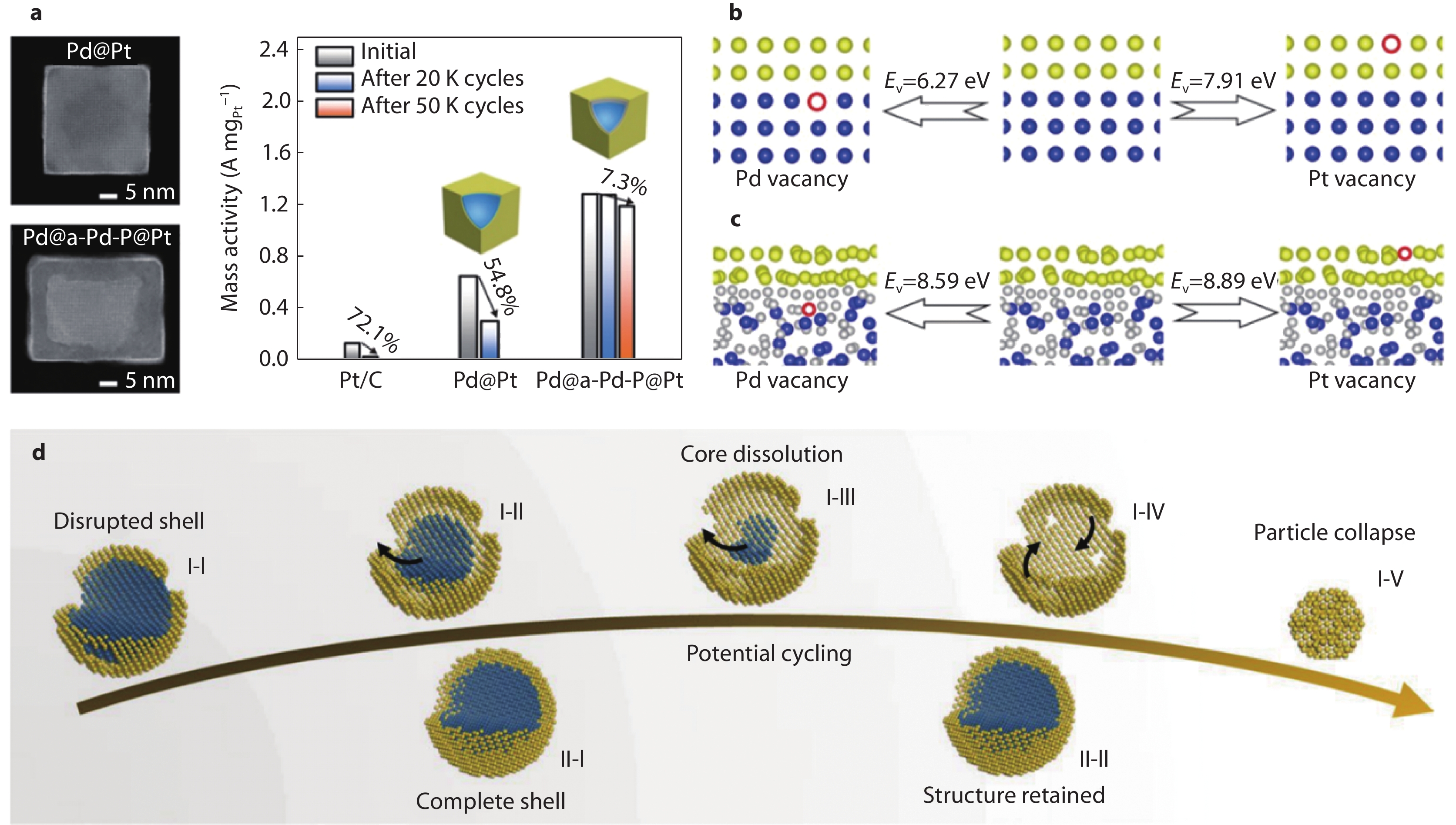
Figure 8.
a TEM images of Pd@Pt and Pd@a-Pd-P@Pt and the corresponding mass activity upon potential cycles. b Theoretical models showing the formation energies of Pt vacancy and Pd vacancy for Pd@Pt2L. c Theoretical models showing the formation energies of Pt vacancy and Pd vacancy for Pd@a-Pd-P@Pt2L.[71] Copyright 2021, American Chemical Society. d Schematic showing the evolution of the partially and fully covered core-shell particles during potential cycling.[11] Copyright 2019, Springer Nature Limited.

 Yuliang Yuan is now an associate professor at Hainan University. He received his bachelor’s degree in materials chemistry from China University of Geosciences (Wuhan) in 2012 and his Ph.D. degree in materials science and engineering from Zhejiang University in 2018. After graduation, he joined Hunan University as a postdoctoral fellow in 2019. Then he studied in University of Wisconsin-Madison as a visiting scholar in 2020. His research interests include the design of materials related to electrochemical energy storage and electrochemical catalysis.
Yuliang Yuan is now an associate professor at Hainan University. He received his bachelor’s degree in materials chemistry from China University of Geosciences (Wuhan) in 2012 and his Ph.D. degree in materials science and engineering from Zhejiang University in 2018. After graduation, he joined Hunan University as a postdoctoral fellow in 2019. Then he studied in University of Wisconsin-Madison as a visiting scholar in 2020. His research interests include the design of materials related to electrochemical energy storage and electrochemical catalysis.  Xuli Chen is an associate professor at Hunan University. She earned her Ph.D. degree in 2014 at Fudan University and completed her postdoctoral training at Case Western Reserve University in 2016. Her research focuses on the development of advanced nanomaterials and their applications in electrochemistry.
Xuli Chen is an associate professor at Hunan University. She earned her Ph.D. degree in 2014 at Fudan University and completed her postdoctoral training at Case Western Reserve University in 2016. Her research focuses on the development of advanced nanomaterials and their applications in electrochemistry.  Hongwen Huang is now a full professor at Hunan University. He received his bachelor’s degree from South China University of Technology in 2009 and Ph.D. degree in materials science and engineering from Zhejiang University in 2015. During 2012–2014, he studied in Georgia Institute of Technology under the supervision of Prof. Younan Xia. After graduation, he joined University of Science and Technology of China as a postdoctoral fellow from 2015 to 2017 and moved to Hunan University in 2017. His research interests include the controlled growth of nanocrystals and their applications in energy-related electrocatalysis.
Hongwen Huang is now a full professor at Hunan University. He received his bachelor’s degree from South China University of Technology in 2009 and Ph.D. degree in materials science and engineering from Zhejiang University in 2015. During 2012–2014, he studied in Georgia Institute of Technology under the supervision of Prof. Younan Xia. After graduation, he joined University of Science and Technology of China as a postdoctoral fellow from 2015 to 2017 and moved to Hunan University in 2017. His research interests include the controlled growth of nanocrystals and their applications in energy-related electrocatalysis. 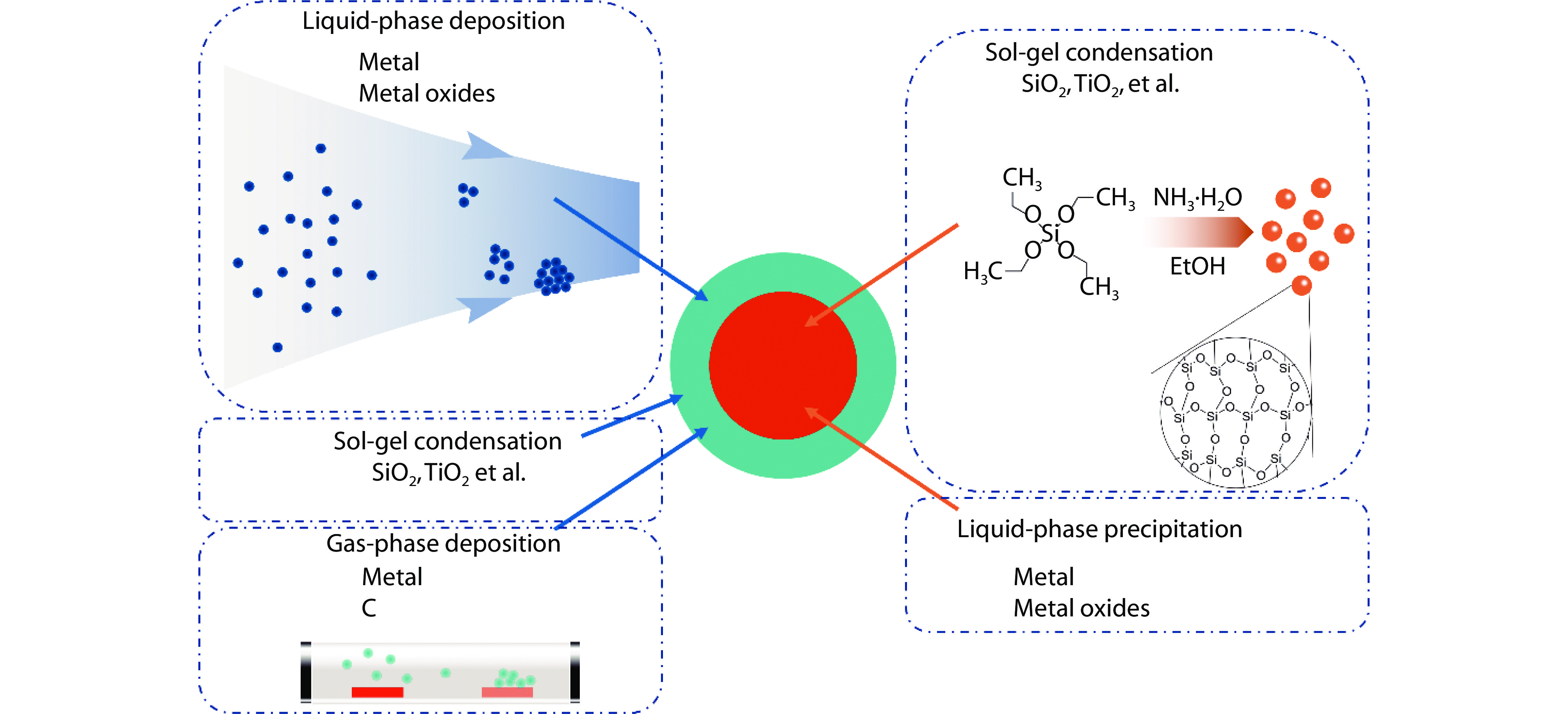

 DownLoad:
DownLoad: