| Citation: | Yijiang Chen, Shan Yu, Jiahao Zhao, Yihan Sun, Xinxin Lu, Zebiao Li, Ying Zhou. Recent advance in solar-driven photothermal water-splitting for hydrogen production: a minireview[J]. Materials Lab, 2025, 4(2): 240009. doi: 10.54227/mlab.20240009 |
Recent advance in solar-driven photothermal water-splitting for hydrogen production: a minireview
-
Abstract
Solar energy's potential to revolutionize the energy sector is undeniable, with its ability to produce hydrogen—a clean and renewable fuel—via solar-driven catalytic water splitting. This review highlights the critical strides in solar-driven photothermal catalysis for hydrogen production, a technology that is promising, eco-friendly, and sustainable for energy in future. It first discusses in detail the classification of solar-driven photothermal catalytic water splitting for hydrogen production and comprehensively reviews the recent progresses, specifically dividing them into photothermal catalysis under external heating, photothermal catalysis by the concentrated sunlight, and photothermal catalysis using the full spectrum of unconcentrated sunlight for hydrogen production. Further, the mechanism of introducing thermal energy to enhance photocatalytic hydrogen production efficiency was also discussed, focusing on five aspects: thermodynamics, ionization constant, charge carriers, mass transfer process, and hydrogen-oxygen recombination reaction. Finally, this review summarizes potential challenges and possible research directions of solar-driven photothermal catalytic hydrogen production, aiming to provide readers with research clues and promote the further development of photothermal catalytic technology in hydrogen production.
-
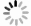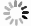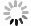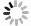
-
References
1. S. Mohanty, P. K. Patra, S. S. Sahoo and A. Mohanty, Renewable and Sustainable Energy Reviews, 2017, 78, 539 2. F. A. Chowdhury, M. L. Trudeau, H. Guo and Z. Mi, Nature Communications, 2018, 9, 1707 3. Q. Wang, M. Nakabayashi, T. Hisatomi, S. Sun, S. Akiyama, Z. Wang, Z. Pan, X. Xiao, T. Watanabe, T. Yamada, N. Shibata, T. Takata and K. Domen, Nature Materials, 2019, 18, 827 4. K.-H. Ye, H. Li, D. Huang, S. Xiao, W. Qiu, M. Li, Y. Hu, W. Mai, H. Ji and S. Yang, Nature Communications, 2019, 10, 3687 5. S. Guo, X. Li, J. Li and B. Wei, Nature Communications, 2021, 12, 1343 6. S. Yu, Y. Li, A. Jiang, Y. Chen, Y. Duan, J. Ye and Y. Zhou, Advanced Energy Materials, 2024, 14, 2304362 7. C. Wu, K. Lv, X. Li and Q. Li, Chinese Journal of Catalysis, 2023, 54, 137 8. Y. Chen, S. Yu, Y. Zhong, Y. Wang, J. Ye and Y. Zhou, Processes, 2023, 11, 3160 9. Y. Zhang, Y. Xiao, S. Abuelgasim and C. Liu, Clean Energy Science and Technology, 2024, 2, 117 10. X. Chu, C. I. Sathish, M. Li, J.-H. Yang, W. Li, D.-C. Qi, D. Chu, A. Vinu and J. Yi, Battery Energy, 2023, 2, 20220041 11. Y. Wang, W. Huang, S. Guo, X. Xin, Y. Zhang, P. Guo, S. Tang and X. Li, Advanced Energy Materials, 2021, 11, 2102452 12. Z. Wang, Y. Luo, T. Hisatomi, J. J. M. Vequizo, S. Suzuki, S. Chen, M. Nakabayashi, L. Lin, Z. Pan, N. Kariya, A. Yamakata, N. Shibata, T. Takata, K. Teshima and K. Domen, Nature Communications, 2021, 12, 1005 13. J. Li, L. Ding, Z. Su, K. Li, F. Fang, R. Sun, Y. Qin and K. Chang, Advanced Materials, 2023, 35, 2305535 14. L. Jiao, Y. Dong, X. Xin, L. Qin and H. Lv, Applied Catalysis B: Environmental, 2021, 291, 120091 15. L. Liao, Q. Zhang, Z. Su, Z. Zhao, Y. Wang, Y. Li, X. Lu, D. Wei, G. Feng, Q. Yu, X. Cai, J. Zhao, Z. Ren, H. Fang, F. Robles-Hernandez, S. Baldelli and J. Bao, Nature Nanotechnology, 2014, 9, 69 16. X. Yan, M. Xia, H. Liu, B. Zhang, C. Chang, L. Wang and G. Yang, Nature Communications, 2023, 14, 1741 17. Z. Wang, C. Li and K. Domen, Chemical Society Reviews, 2019, 48, 2109 18. S. Chen, T. Takata and K. Domen, Nature Reviews Materials, 2017, 2, 17050 19. P. Varadhan, H.-C. Fu, Y.-C. Kao, R.-H. Horng and J.-H. He, Nature Communications, 2019, 10, 5282 20. S. Fang, Z. Sun and Y. H. Hu, ACS Catalysis, 2019, 9, 5047 21. S. Fang, Y. Liu, Z. Sun, J. Lang, C. Bao and Y. H. Hu, Applied Catalysis B: Environmental, 2020, 278, 119316 22. Y. Li, H. Zhou, S. Cai, D. Prabhakaran, W. Niu, A. Large, G. Held, R. A. Taylor, X.-P. Wu and S. C. E. Tsang, Nature Catalysis, 2024, 7, 77 23. Y. Shen, Y. Shi, Z. Chen, S. Zhang, K. Chen, X. Luo, F. Guo, G. Wang and W. Shi, Chemical Engineering Journal, 2024, 484, 149607 24. X. Zhu, Y. Liu, M. Wang, L. Zhang, Q. Li, E. Zhang, H. Mo, Y. Gao, C. Xu and Y. Zhang, Chemical Engineering Journal, 2024, 479, 147636 25. S. Song, H. Song, L. Li, S. Wang, W. Chu, K. Peng, X. Meng, Q. Wang, B. Deng, Q. Liu, Z. Wang, Y. Weng, H. Hu, H. Lin, T. Kako and J. Ye, Nature Catalysis, 2021, 4, 1032 26. L. Zhou, J. M. P. Martirez, J. Finzel, C. Zhang, D. F. Swearer, S. Tian, H. Robatjazi, M. Lou, L. Dong, L. Henderson, P. Christopher, E. A. Carter, P. Nordlander and N. J. Halas, Nature Energy, 2020, 5, 61 27. B. Han, W. Wei, L. Chang, P. Cheng and Y. H. Hu, ACS Catalysis, 2016, 6, 494 28. D. Takami, J. Tsubakimoto, W. Sarwana, A. Yamamoto and H. Yoshida, ACS Applied Energy Materials, 2023, 6, 7627 29. C. Sun, K. Zhao and Z. Yi, Journal of Inorganic Materials, 2023, 38, 1245 30. C. Wang, S. Fang, S. Xie, Y. Zheng and Y. H. Hu, Journal of Materials Chemistry A, 2020, 8, 7390 31. T. H. Tan, B. Xie, Y. H. Ng, S. F. B. Abdullah, H. Y. M. Tang, N. Bedford, R. A. Taylor, K.-F. Aguey-Zinsou, R. Amal and J. Scott, Nature Catalysis, 2020, 3, 1034 32. M. Cai, Z. Wu, Z. Li, L. Wang, W. Sun, A. A. Tountas, C. Li, S. Wang, K. Feng, A.-B. Xu, S. Tang, A. Tavasoli, M. Peng, W. Liu, A. S. Helmy, L. He, G. A. Ozin and X. Zhang, Nature Energy, 2021, 6, 807 33. X. Shi, W. Peng, Y. Huang, C. Gao, Y. Fu, Z. Wang, L. Yang, Z. Zhu, J. Cao, F. Rao, G. Zhu, S. Lee and Y. Xiong, Nature Communications, 2024, 15, 10135 34. Y. Wan, F. Fang, R. Sun, J. Zhang and K. Chang, Acta Physico-Chimica Sinica, 2023, 39, 2212042 35. L. Zhao, Y. Qi, L. Song, S. Ning, S. Ouyang, H. Xu and J. Ye, Angewandte Chemie International Edition, 2019, 58, 7708 36. Z. Li, J. Liu, Y. Zhao, G. I. N. Waterhouse, G. Chen, R. Shi, X. Zhang, X. Liu, Y. Wei, X.-D. Wen, L.-Z. Wu, C.-H. Tung and T. Zhang, Advanced Materials, 2018, 30, 1800527 37. U. Aslam, S. Chavez and S. Linic, Nature Nanotechnology, 2017, 12, 1000 38. Z. Liu, L. Niu, X. Zong, L. An, D. Qu, X. Wang and Z. Sun, Applied Catalysis B: Environmental, 2022, 313, 121439 39. X. Li, X. Zhang, H. O. Everitt and J. Liu, Nano Letters, 2019, 19, 1706 40. Y. Peng, J. Albero, A. Franconetti, P. Concepción and H. García, ACS Catalysis, 2022, 12, 4938 41. T. Hou, L. Chen, Y. Xin, W. Zhu, C. Zhang, W. Zhang, S. Liang and L. Wang, ACS Energy Letters, 2020, 5, 2444 42. Q. Zhu, J. Song, Z. Liu, K. Wu, X. Li, Z. Chen and H. Pang, Journal of Colloid and Interface Science, 2022, 623, 992 43. Q. Yang, G. Tan, B. Zhang, S. Feng, Y. Bi, Z. Wang, A. Xia, H. Ren and W. Liu, Chemical Engineering Journal, 2023, 458, 141378 44. Y. Wang, Y. Zeng, Z. Xiao, P. Chen, S. Huang, Z. Xu, W. Lv and G. Liu, Chemical Engineering Journal, 2024, 501, 157739 45. X. Li, R. Yang, L. Zou, S. Zheng, M. Chen, J. Wen, H. Zhang, C. Wu, Y. Zhang and Y. Zhou, Advanced Materials, 2025, 37, 2416283 46. T. Liu, P. Sun, W. Zhao, Y. Li, L. Cheng, J. Fan, X. Bi and X. Dong, Chinese Chemical Letters, 2024, 35, 108813 47. J. Huang, Z. Huang, T. Liu, Y. Wen, J. Yuan, S. Yang and H. Li, Chinese Chemical Letters, 2025, 36, 110179 48. T. Li, P.-L. Zhang, L.-Z. Dong and Y.-Q. Lan, Angewandte Chemie International Edition, 2024, 63, e202318180 49. A. Marimuthu, J. Zhang and S. Linic, Science, 2013, 339, 1590 50. Y. Wei, D. Chen, X. Fan, X. Tang, L. Yao, X. Zhao, Q. Li, J. Wang, T. Qiu and Q. Hao, ACS Catalysis, 2024, 14, 15043 51. F. Wang, C. Li, H. Chen, R. Jiang, L.-D. Sun, Q. Li, J. Wang, J. C. Yu and C.-H. Yan, Journal of the American Chemical Society, 2013, 135, 5588 52. H. L. Yajin Li, Lan Ma, Jiaxiong Liu, Dehua He, Acta Physico-Chimica Sinica, 2024, 40, 2308005 53. H. Song, S. Luo, H. Huang, B. Deng and J. Ye, ACS Energy Letters, 2022, 7, 1043 54. J. Zhang, Z. Hu, J. Zheng, Y. Xiao, J. Song, X. Li, C. Cheng and Z. Zhang, Energy Conversion and Management, 2024, 318, 118901 55. S. Fang and Y. H. Hu, Chemical Society Reviews, 2022, 51, 3609 56. B. Han and Y. H. Hu, The Journal of Physical Chemistry C, 2015, 119, 18927 57. H. Nishiyama, T. Yamada, M. Nakabayashi, Y. Maehara, M. Yamaguchi, Y. Kuromiya, Y. Nagatsuma, H. Tokudome, S. Akiyama, T. Watanabe, R. Narushima, S. Okunaka, N. Shibata, T. Takata, T. Hisatomi and K. Domen, Nature, 2021, 598, 304 58. J. H. Kim, D. Hansora, P. Sharma, J.-W. Jang and J. S. Lee, Chemical Society Reviews, 2019, 48, 1908 59. B. D. Sherman, N. K. McMillan, D. Willinger and G. Leem, Nano Convergence, 2021, 8, 7 60. J.-W. Lee, K.-H. Cho, J.-S. Yoon, Y.-M. Kim and Y.-M. Sung, Journal of Materials Chemistry A, 2021, 9, 21576 61. Z. Yu, H. Liu, M. Zhu, Y. Li and W. Li, Small, 2021, 17, 1903378 62. N. Pirrone, F. Bella and S. Hernández, Green Chemistry, 2022, 24, 5379 63. F. Dingenen and S. W. Verbruggen, Renewable and Sustainable Energy Reviews, 2021, 142, 110866 64. A. Vilanova, P. Dias, T. Lopes and A. Mendes, Chemical Society Reviews, 2024, 53, 2388 65. Q. Wang and K. Domen, Chemical Reviews, 2020, 120, 919 66. X. Chen, C. Li, M. Grätzel, R. Kostecki and S. S. Mao, Chemical Society Reviews, 2012, 41, 7909 67. A. Kudo and Y. Miseki, Chemical Society Reviews, 2009, 38, 253 68. Q. Yang, X. Tong and Z. Wang, Materials Reports: Energy, 2024, 4, 100253 69. L. Wang, Y. Zhang, W. Li and L. Wang, Materials Reports: Energy, 2023, 3, 100232 70. P. Zhu, Y. Shi, C. Lou, C. Zeng and C. Wang, Low-Carbon Chemistry and Chemical Engineering, 2022, 47, 145 71. G. Yang, Q. Xu and G. Zeng, Electron, 2024, 2, e39 72. W. Zhang, Y. Chao and S. Guo, Energy Lab, 2023, 1, 220004 73. X. Liang, Q. Wu, Q. Liu, L. Wang, M. Zhang, K. Sun, Y. Shen, H. Chen and X. Zou, Energy Lab, 2023, 1, 220013 74. T. Wang, C. Duanmu, X. Meng, J. Zou and Y. Pan, Low-Carbon Chemistry and Chemical Engineering, 2024, 49, 41 75. K. Wang, D. Huang, X. Li, K. Feng, M. Shao, J. Yi, W. He and L. Qiao, Electron, 2023, 1, e4 76. S. Yao, Y. Wei, Z. Lu, S. Guo, J. Chen and Z. Yu, Renewables, 2023, 1, 397 77. S. Docao, A. R. Koirala, M. G. Kim, I. C. Hwang, M. K. Song and K. B. Yoon, Energy & Environmental Science, 2017, 10, 628 78. J. Li, M. Hatami, Y. Huang, B. Luo, D. Jing and L. Ma, International Journal of Hydrogen Energy, 2023, 48, 6336 79. R. Song, M. Liu, B. Luo, J. Geng and D. Jing, AIChE Journal, 2020, 66, e17008 80. X. Zhang, C. Xu, L. Zhang, Z. Li, J. Hong and Y. Zhang, ACS Applied Energy Materials, 2022, 5, 4564 81. L. Zhang, X. Zhang, H. Mo, J. Hong, S. Yang, Z. Zhan, C. Xu and Y. Zhang, ACS Applied Materials & Interfaces, 2023, 15, 39304 82. X. Luo, R. Li, K. P. Homewood, X. Chen and Y. Gao, Applied Surface Science, 2020, 505, 144099 83. R. Song, B. Luo, J. Geng, D. Song and D. Jing, Industrial & Engineering Chemistry Research, 2018, 57, 7846 84. S. Hu, J. Shi, B. Luo, C. Ai and D. Jing, Journal of Colloid and Interface Science, 2022, 608, 2058 85. J. Li, L. Ma, C. Fu, Y. Huang, B. Luo, J. Cao, J. Geng and D. Jing, Industrial & Engineering Chemistry Research, 2022, 61, 6436 86. J. Li, L. Ma, Y. Huang, BingLuo and D. Jing, International Journal of Hydrogen Energy, 2023, 48, 13170 87. R. Li, C. Wen, K. Yan, T. Liu, B. Zhang, M. Xu and Z. Zhou, Journal of Materials Chemistry A, 2023, 11, 7128 88. Y. Li, Y.-K. Peng, L. Hu, J. Zheng, D. Prabhakaran, S. Wu, T. J. Puchtler, M. Li, K.-Y. Wong, R. A. Taylor and S. C. E. Tsang, Nature Communications, 2019, 10, 4421 89. P. Zhou, I. A. Navid, Y. Ma, Y. Xiao, P. Wang, Z. Ye, B. Zhou, K. Sun and Z. Mi, Nature, 2023, 613, 66 90. W. C. Chueh, C. Falter, M. Abbott, D. Scipio, P. Furler, S. M. Haile and A. Steinfeld, Science, 2010, 330, 1797 91. D. Marxer, P. Furler, J. Scheffe, H. Geerlings, C. Falter, V. Batteiger, A. Sizmann and A. Steinfeld, Energy & Fuels, 2015, 29, 3241 92. J. Wang, T. Ming, Z. Jin, J. Wang, L.-D. Sun and C.-H. Yan, Nature Communications, 2014, 5, 5669 93. F. Xue, H. Wu, Y. Liu, M. Min, M. Hatami, N. Li and M. Liu, International Journal of Hydrogen Energy, 2023, 48, 6346 94. X. Sun, Z. Chen, Y. Shen, J. Lu, Y. Shi, Y. Cui, F. Guo and W. Shi, Journal of Colloid and Interface Science, 2023, 652, 1016 95. S. W. L. Ng, M. Gao, W. Lu, M. Hong and G. W. Ho, Advanced Functional Materials, 2021, 31, 2104750 96. R. Xiong, C. Tang, K. Li, J. Wan, W. Jia, Y. Xiao, B. Cheng and S. Lei, Journal of Materials Chemistry A, 2022, 10, 22819 97. S. Zhang, G. Zhang, S. Wu, Z. Guan, Q. Li and J. Yang, Journal of Colloid and Interface Science, 2023, 650, 1974 98. Y. Shi, L. Li, Z. Xu, F. Guo and W. Shi, Chemical Engineering Journal, 2023, 459, 141549 99. X.-Q. Qiao, W. Chen, C. Li, Z. Wang, D. Hou, B. Sun and D.-S. Li, Materials Reports: Energy, 2023, 3, 100234 100. Y. Shi, Z. Chen, P. Hao, P. Shan, J. Lu, F. Guo and W. Shi, Journal of Colloid and Interface Science, 2024, 653, 1339 101. J. Fang, F. Sun, A. Kheradmand, H. Xu, H. Dong, X. Yi, H. Hong and X. Liu, Fuel, 2023, 353, 129277 102. W. H. Lee, C. W. Lee, G. D. Cha, B.-H. Lee, J. H. Jeong, H. Park, J. Heo, M. S. Bootharaju, S.-H. Sunwoo, J. H. Kim, K. H. Ahn, D.-H. Kim and T. Hyeon, Nature Nanotechnology, 2023, 18, 754 103. L. Ding, K. Li, J. Li, Q. Lu, F. Fang, T. Wang and K. Chang, ACS Nano, 2023, 17, 11616 104. C. Pornrungroj, A. B. Mohamad Annuar, Q. Wang, M. Rahaman, S. Bhattacharjee, V. Andrei and E. Reisner, Nature Water, 2023, 1, 952 105. L. He, X. Zeng, H. Chen, L. Zhao, Z. Huang, D. Wang, X. He, W. Fang, X. Du and W. Li, Advanced Functional Materials, 2024, 34, 2313058 106. W. Li, T. Li, B. Deng, T. Xu, G. Wang, W. Hu and C. Si, Advanced Composites and Hybrid Materials, 2024, 7, 52 107. J. Zhang, Y. Shi, X. Huang and X. Qian, Surfaces and Interfaces, 2024, 44, 103689 108. Y. Ren, D. Feng, Z. Yan, Z. Sun, Z. Zhang, D. Xu, C. Qiao, Z. Chen, Y. Jia, S. Chan Jun, S. Liu and Y. Yamauchi, Chemical Engineering Journal, 2023, 453, 139875 109. J. Lu, Y. Shi, Z. Chen, X. Sun, H. Yuan, F. Guo and W. Shi, Chemical Engineering Journal, 2023, 453, 139834 110. Z. Chen, Y. Yan, K. Sun, L. Tan, F. Guo, X. Du and W. Shi, Journal of Colloid and Interface Science, 2024, 661, 12 111. H. Han and X. Meng, Journal of Colloid and Interface Science, 2023, 650, 846 112. Z. Ma, W. Liu, W. Yang, W. Li and B. Han, Fuel, 2021, 286, 119490 113. W. Shi, Z. Chen, J. Lu, X. Sun, Z. Wang, Y. Yan, F. Guo, L. Chen and G. Wang, Chemical Engineering Journal, 2023, 474, 145690 114. C. K. N. Peh, M. Gao and G. W. Ho, Journal of Materials Chemistry A, 2015, 3, 19360 115. W. H. Leng, P. R. F. Barnes, M. Juozapavicius, B. C. O’Regan and J. R. Durrant, The Journal of Physical Chemistry Letters, 2010, 1, 967 116. J. Zheng, L. Lu, K. Lebedev, S. Wu, P. Zhao, I. J. McPherson, T.-S. Wu, R. Kato, Y. Li, P.-L. Ho, G. Li, L. Bai, J. Sun, D. Prabhakaran, R. A. Taylor, Y.-L. Soo, K. Suenaga and S. C. E. Tsang, Chem Catalysis, 2021, 1, 162 117. P. Cheng, D. Wang and P. Schaaf, Advanced Sustainable Systems, 2022, 6, 2200115 118. B. Wang, Y. Si, M. Du, S. Zhao, J. Huang, X. Zhao, S. Wang, K. Lu and M. Liu, Catalysis Science & Technology, 2024, 14, 4646 119. Y. Fu, K. Lu, Y. Wang, Y. Si, J. Shi, N. Li, Z. Zhou and M. Liu, Science Bulletin, 2024, 69, 1833 120. Y. Xiao, M. Li, H. Li, Z. Wang and Y. Wang, Nano Energy, 2024, 120, 109164 121. X. Zang, Y. Qin, M. Gu, Y. Sun, D. Huang, J. Ji and M. Xue, Materials Today Sustainability, 2023, 24, 100558 122. J. Qu, S. Li, B. Zhong, Z. Deng, Y. Shu, X. yang, Y. Cai, J. Hu and C. M. Li, Nanoscale, 2023, 15, 2455 123. J. Zhu, L. Huang, F. Bao, G. Chen, K. Song, Z. Wang, H. Xia, J. Gao, Y. Song, C. Zhu, F. Lu, T. Zheng and M. Ji, Materials Reports: Energy, 2024, 4, 100245 124. J. Wang, Y. Zhang, S. Jiang, C. Sun and S. Song, Angewandte Chemie International Edition, 2023, 62, e202307808 125. X. Zhang, X. Li, M. E. Reish, D. Zhang, N. Q. Su, Y. Gutiérrez, F. Moreno, W. Yang, H. O. Everitt and J. Liu, Nano Letters, 2018, 18, 1714 126. Y. Zheng, L. Zhang, J. Guan, S. Qian, Z. Zhang, C. K. Ngaw, S. Wan, S. Wang, J. Lin and Y. Wang, ACS Sustainable Chemistry & Engineering, 2021, 9, 1754 127. F. Dionigi, P. C. K. Vesborg, T. Pedersen, O. Hansen, S. Dahl, A. Xiong, K. Maeda, K. Domen and I. Chorkendorff, Energy & Environmental Science, 2011, 4, 2937 128. S. I. Nikitenko, T. Chave, C. Cau, H.-P. Brau and V. Flaud, ACS Catalysis, 2015, 5, 4790 129. A. Ruiz-Aguirre, J. G. Villachica-Llamosas, M. I. Polo-López, A. Cabrera-Reina, G. Colón, J. Peral and S. Malato, Energy, 2022, 260, 125199 130. Q. Wei, Y. Yang, J. Hou, H. Liu, F. Cao and L. Zhao, Solar Energy, 2017, 153, 215 131. Z. Kuspanov, B. Bakbolat, A. Baimenov, A. Issadykov, M. Yeleuov and C. Daulbayev, Science of The Total Environment, 2023, 885, 163914 -
Rights and permissions
This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.
Information
Article Metrics
-
Figure 1.
Schematic diagram of photothermal catalytic water splitting for hydrogen production classification.
-
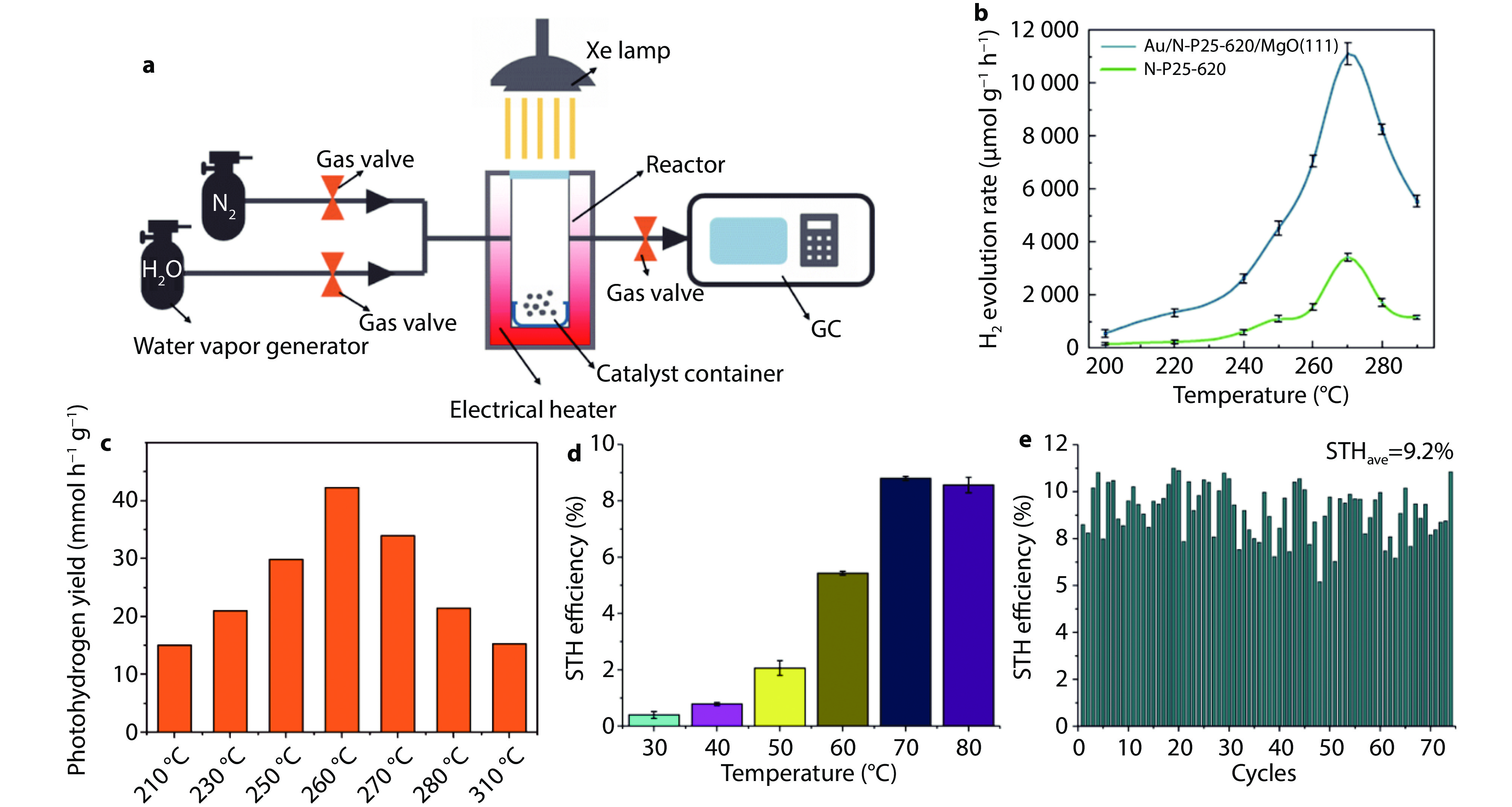
Figure 2.
Photothermal catalytic water splitting for hydrogen production under external heating. a Schematic diagram of the reaction system;[87] Copyright 2023, Royal Society of Chemistry. b The activity of photothermal catalytic water splitting for H2 production at different temperatures over N-TiO2 (P25)-620 and Au/N-P25-620/MgO(111), 620 represents the treatment temperature of P25 in ammonia is 620 °C (300 W Xe lamp).[88] Copyright 2019, Springer Nature. c Hydrogen production rates over Rh-loaded TiO2 at various temperatures. (Xe lamp equipped with an AM 1.5G filter, at a light intensity of 100 mW cm−1[2]).[21] Copyright 2020, Elsevier. d STH at various temperatures over Rh/Cr2O3/Co3O4–InGaN/GaN NWs (concentrated light intensity of 3.8 W cm−1[2]); e Stability testing over Rh/Cr2O3/Co3O4–InGaN/GaN nanowires by external heating at 70 °C (with concentrated light intensity of 3.8 W cm−1[2]).[89] Copyright 2023, Springer Nature.
-
Figure 3.
Photothermal catalytic water splitting for H2 production by the concentrated sunlight. a Physical image of the reaction device, a 1.1 m × 1.1 m Fresnel lens produced concentrated natural light of approximately 16.07 W cm−1[2] in a plane area of about 8 cm × 8 cm; b Production efficiency of H2, O2, and STH result of the reaction in a) with InGaN/GaN nanowires supported on Rh/Cr2O3/Co3O4 as the photocatalyst.[89] Copyright 2023, Springer Nature. c Specially designed reactor (achieved light-concentrated furnace by a four-mirror floating-zone light furnace) for the photothermal catalytic water splitting for H2 production reaction; d The STH efficiency results at 270 °C with the reactor in c) over Pt/N-TiO2 in Dead Sea water.[22] Copyright 2024, Springer Nature.
-
Figure 4.
H2 production efficiency and surface temperature distribution of different samples under UV-Vis and UV-Vis-IR (full spectrum) irradiation. a H2 production efficiency of ST NS, AST NS, and ST NS-Au); b Hydrogen production efficiency of ST NS and AST NS at 60 °C; c Surface temperature distribution of SiO2 and AS.[95] Copyright 2021, Wiley-VCH. d Photothermal images of CN, CCO and CCO/CN with different CCO amounts after 300 s of irradiation; e H2 production efficiency of them.[96] Copyright 2022, Royal Society of Chemistry. f-g The heat transfer processes 3% Ag2S/PCNVs: f A two-dimensional (2D) finite element model at 20 °C; g A 2D finite element model at 59.6 °C; h photothermal conversion efficiency of 3% Ag2S/PCNVs.[94] Copyright 2023, Elsevier.
-
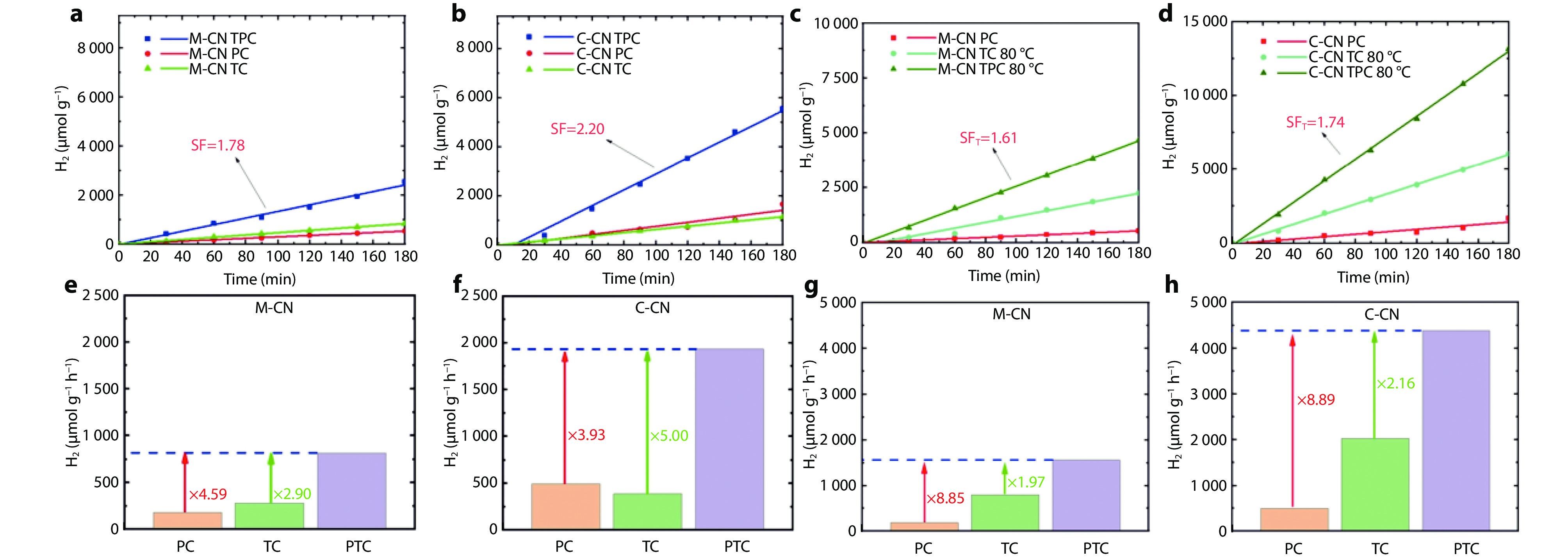
Figure 5.
Evaluation of light and thermal effects on hydrogen production activity with M-CN and C-CN under different conditions. a-b M-CN (a) and C-CN (b) under full spectrum irradiation by PTC (60 °C), PC, and TC (60 °C) methods; c-d M-CN (c) and C-CN (d) under external heating with different methods (PTC (80 °C), PC, and TC (80 °C)); e-f M-CN (e) and C-CN (f) under full spectrum irradiation; g-h M-CN (g) and C-CN (h) under full spectrum irradiation.[101] Copyright 2023, Elsevier.
-
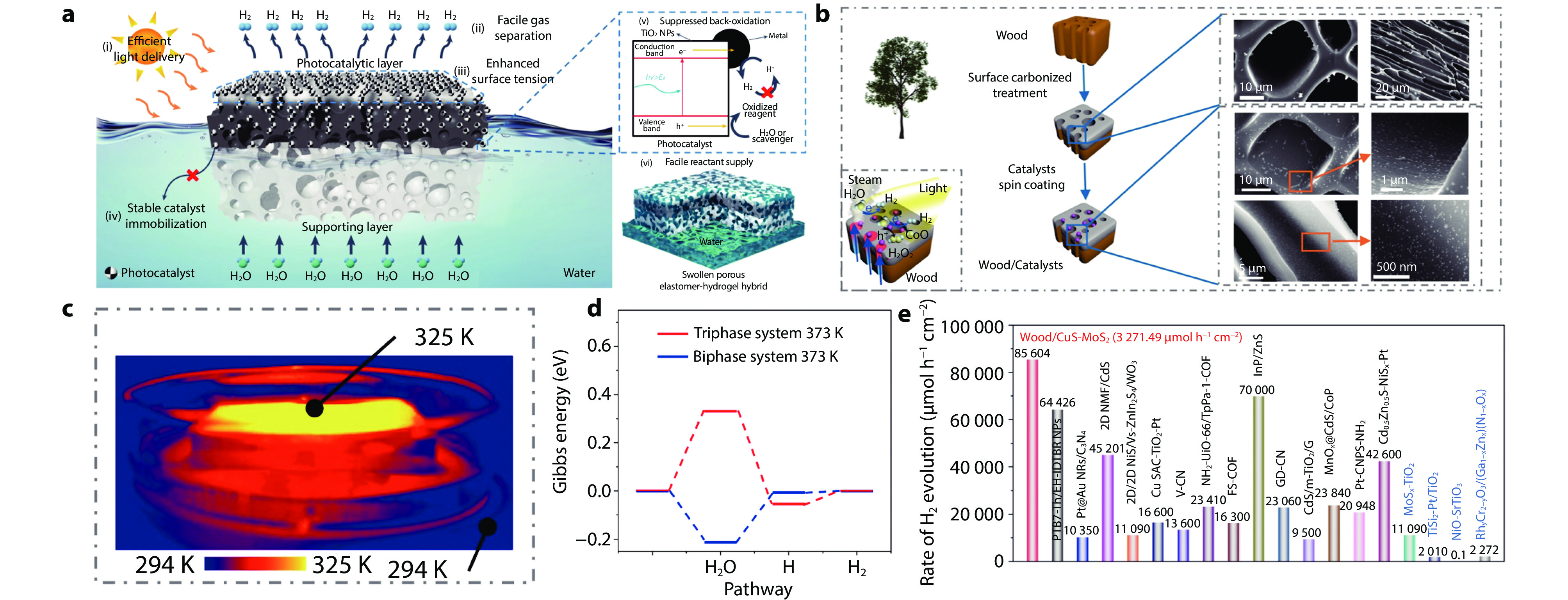
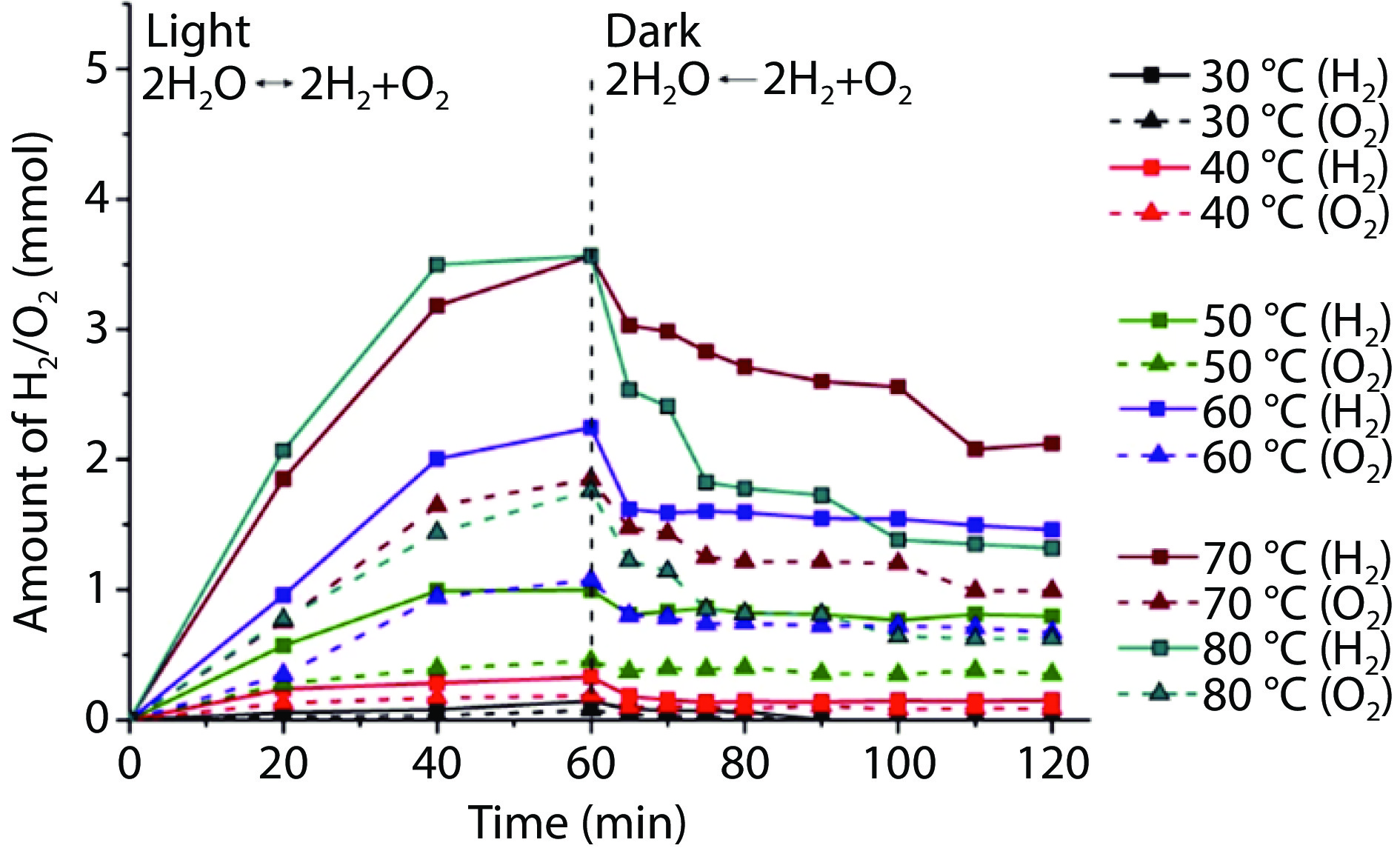
Figure 6.
Powder 3D assembled catalytic system of photothermal catalytic water splitting for hydrogen production under irradiation without concentrated light. a Structural diagram of porous elastomer hydrogel nanocomposite.[102] Copyright 2023, Springer Nature. b Schematic structure and microscopic images of wood/CoO composite; c thermal imaging of Wood/CoO composite under light irradiation; d Gibbs free energy changes of different phase interfaces for hydrogen evolution from water splitting; e comparison of the photocatalytic water splitting efficiency of carbonized wood/CuS-MoS2 with others (solar simulator (AM 1.5 G), 100 mW cm−1[2]).[5] Copyright 2021, Springer Nature.
-
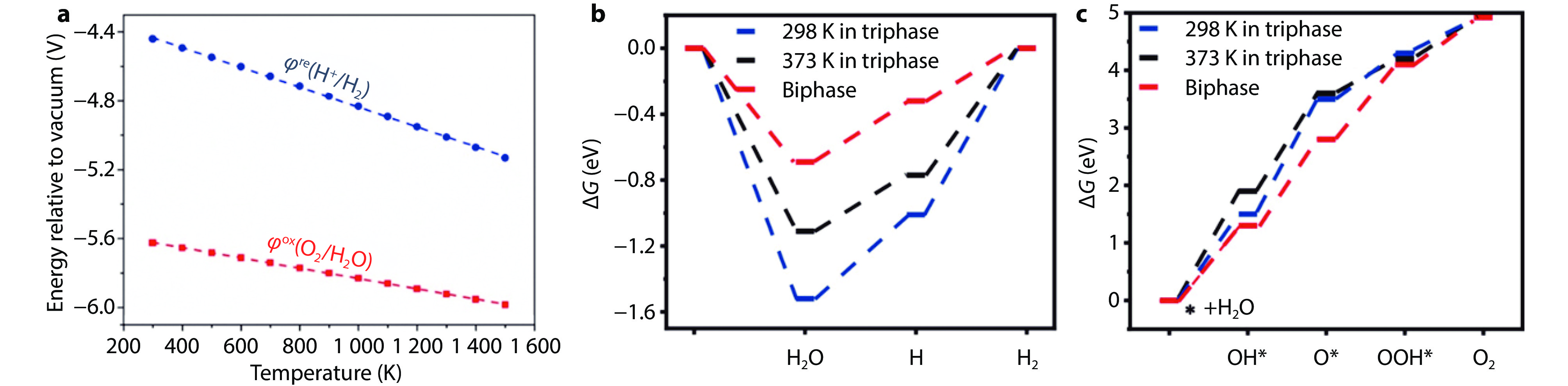
Figure 7.
Redox potential and Gibbs free energy (ΔG) changes of water splitting at different temperatures. a Temperature dependence of redox potential of half-reaction in water splitting.[112] Copyright 2021, Elsevier. b-c The (ΔG) changes for H2 (b) and O2 (c) evolution in the biphase and triphase system at different temperatures.[11] Copyright 2021, Wiley-VCH.
-
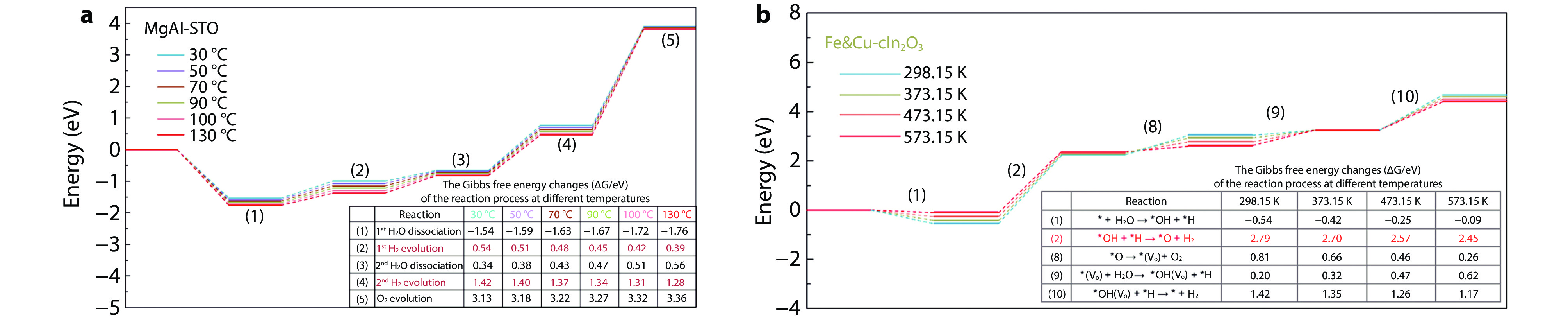
Figure 8.
The reaction energy barriers of different catalysts in photothermal catalytic water splitting for H2 production at different temperatures. a Mg, Al co-doped and Rh/Cr2O3/CoOOH co-loaded SrTiO3.[24] Copyright 2024, Elsevier. b Fe&Cu-In2O3.[81] Copyright 2023, American Chemical Society.
-
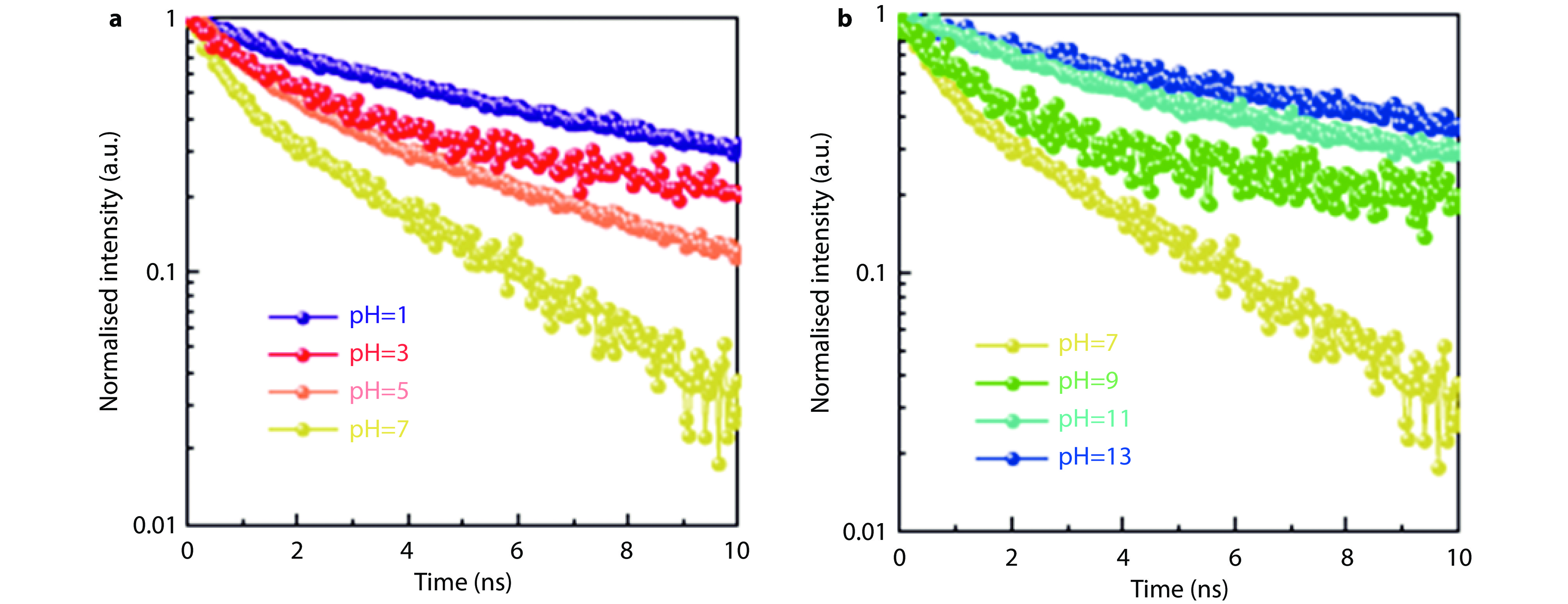
Figure 9.
TRPL decay of N-TiO2 at different pH environments (simulate different ionization constants). a Acidic conditions; b alkaline conditions.[88] Copyright 2019, Springer Nature.
-
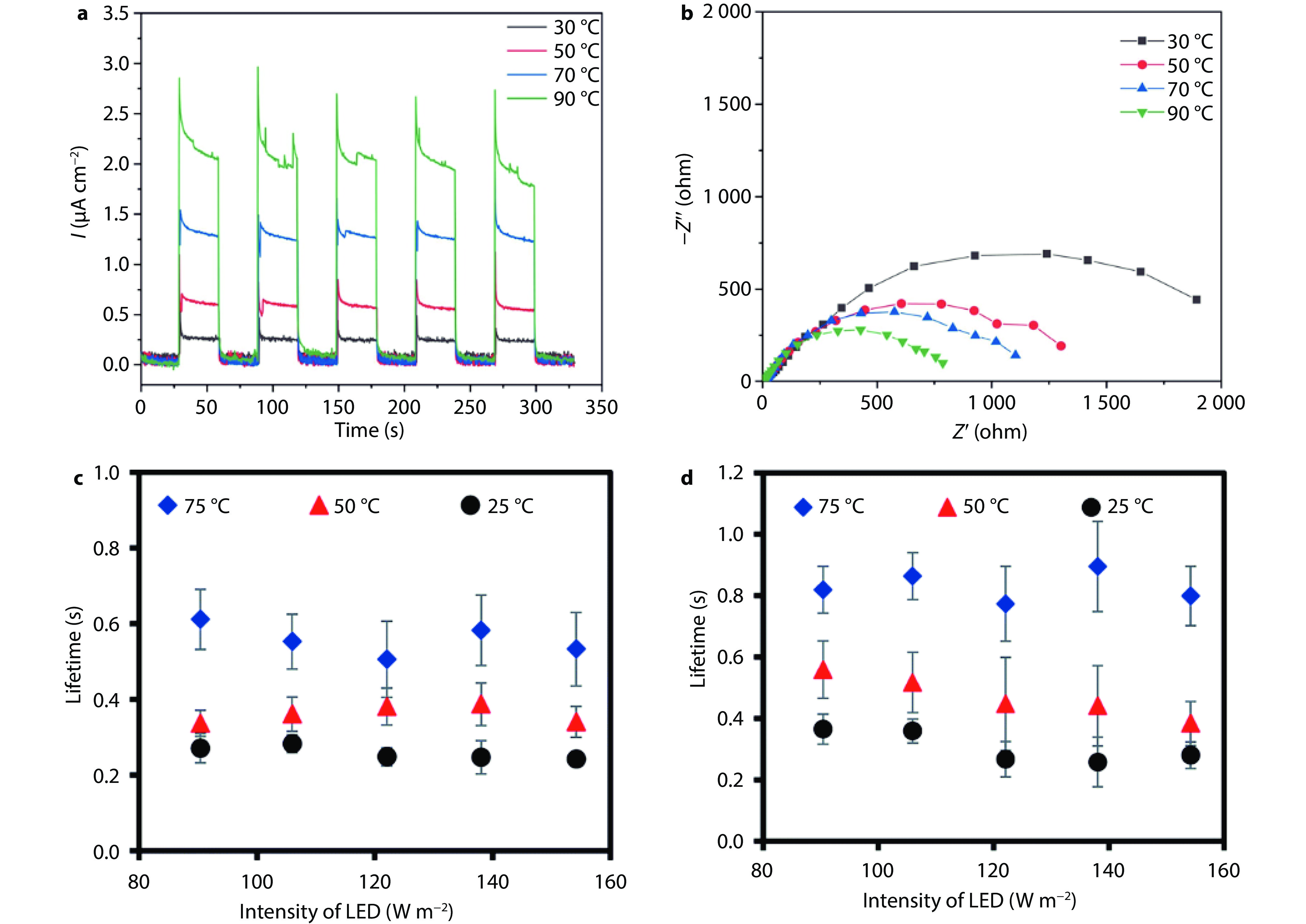
Figure 10.
Influence of temperature on the charge carrier transfer process. a Photocurrent results and b EIS results of Mg, Al co-doped and Rh/Cr2O3/CoOOH co-loaded SrTiO3 at different temperatures.[24] Copyright 2024, Elsevier. c-d The lifetime of photogenerated electrons by transient photovoltage measurements of TiO2 NPs (c) and TiO2 NTs (d) at different temperatures.[114] Copyright 2015, Royal Society of Chemistry.
-
Figure 11.
Experimental study on the temperature dependence of hydrogen-oxygen recombination reaction over Rh/Cr2O3/Co3O4–InGaN/GaN nanowires (with concentrated light intensity of 3.8 W cm−1[2]).[89] Copyright 2023, Springer Nature.
-
Figure 12.
The schematic diagram for the potential effects on the improved activity of the photothermal catalytic water splitting mechanism for H2 production: ① thermodynamics and reaction activation energy; ② ionization constant (H+ concentration); ③ charge transfer of carriers; ④ mass transfer process; ⑤ hydrogen-oxygen recombination reaction.

 Yijiang Chen received his BSc degree from Southwest Petroleum University, China in 2020. Currently he is a Ph.D. candidate at Southwest Petroleum University with Prof. Ying Zhou. His present research interests include photothermal catalytic water splitting for H2 production.
Yijiang Chen received his BSc degree from Southwest Petroleum University, China in 2020. Currently he is a Ph.D. candidate at Southwest Petroleum University with Prof. Ying Zhou. His present research interests include photothermal catalytic water splitting for H2 production.  Shan Yu received her BSc and Ph.D. degree from Lanzhou University and Technical Institute of Physics and Chemistry, Chinese Academy of Sciences in 2009 and 2014, respectively. She was also a guest Postdoc from 2016 to 2017 at the University of Zurich. Currently, she is a professor at Southwest Petroleum University, China. Her research interests include solar-driven hydrogen production and spectroscopic studies of photocatalysts.
Shan Yu received her BSc and Ph.D. degree from Lanzhou University and Technical Institute of Physics and Chemistry, Chinese Academy of Sciences in 2009 and 2014, respectively. She was also a guest Postdoc from 2016 to 2017 at the University of Zurich. Currently, she is a professor at Southwest Petroleum University, China. Her research interests include solar-driven hydrogen production and spectroscopic studies of photocatalysts.  Jiahao Zhao is currently a postgraduate student in Prof. Ying Zhou's research group at Southwest Petroleum University, China. His current research mainly focuses on photocatalytic water splitting using heterojunction catalysts.
Jiahao Zhao is currently a postgraduate student in Prof. Ying Zhou's research group at Southwest Petroleum University, China. His current research mainly focuses on photocatalytic water splitting using heterojunction catalysts.  Yihan Sun is currently a postgraduate student in Ying Zhou's research group at Southwest Petroleum University. His current research mainly focuses on photothermal catalytic water splitting for hydrogen production.
Yihan Sun is currently a postgraduate student in Ying Zhou's research group at Southwest Petroleum University. His current research mainly focuses on photothermal catalytic water splitting for hydrogen production.  Xinxin Lu received her Ph.D. degree in Particles and Catalysis Research Group at the University of New South Wales, and did a postdoctoral research at School of Science and Engineering of the Chinese University of Hong Kong (Shenzhen). Her research mainly focuses on the fabrication of heterogeneous catalysts, theoretical simulations on catalyst properties, and photo(electro)catalysis for energy conversion applications.
Xinxin Lu received her Ph.D. degree in Particles and Catalysis Research Group at the University of New South Wales, and did a postdoctoral research at School of Science and Engineering of the Chinese University of Hong Kong (Shenzhen). Her research mainly focuses on the fabrication of heterogeneous catalysts, theoretical simulations on catalyst properties, and photo(electro)catalysis for energy conversion applications.  Zebiao Li received his Ph.D. degree from the department of Materials Science and Engineering of City University of Hong Kong in 2020. He is currently an assistant research fellow at PetroChina ShenZhen New Energy Research Institute Co., LTD. His research interests include photo/photoelectric hydrogen production.
Zebiao Li received his Ph.D. degree from the department of Materials Science and Engineering of City University of Hong Kong in 2020. He is currently an assistant research fellow at PetroChina ShenZhen New Energy Research Institute Co., LTD. His research interests include photo/photoelectric hydrogen production.  Ying Zhou received his BSc and MSc from Central South University and the Chinese Academy of Sciences, respectively. In 2010, he received his Ph.D. at the University of Zurich (UZH). He then continued his work with a postdoctoral Forschungskredit grant from UZH. He was also awarded a fellowship by the Alexander von Humboldt Foundation at Karlsruhe Institute of Technology and was a visiting professor at Kyoto University. He currently holds a professorship at Southwest Petroleum University, China. His research mainly focuses on the integrated development of new energy and fossil energy such as clean hydrogen generation from the gas waste from fossil energy, as well as the related in situ characterization techniques.
Ying Zhou received his BSc and MSc from Central South University and the Chinese Academy of Sciences, respectively. In 2010, he received his Ph.D. at the University of Zurich (UZH). He then continued his work with a postdoctoral Forschungskredit grant from UZH. He was also awarded a fellowship by the Alexander von Humboldt Foundation at Karlsruhe Institute of Technology and was a visiting professor at Kyoto University. He currently holds a professorship at Southwest Petroleum University, China. His research mainly focuses on the integrated development of new energy and fossil energy such as clean hydrogen generation from the gas waste from fossil energy, as well as the related in situ characterization techniques. 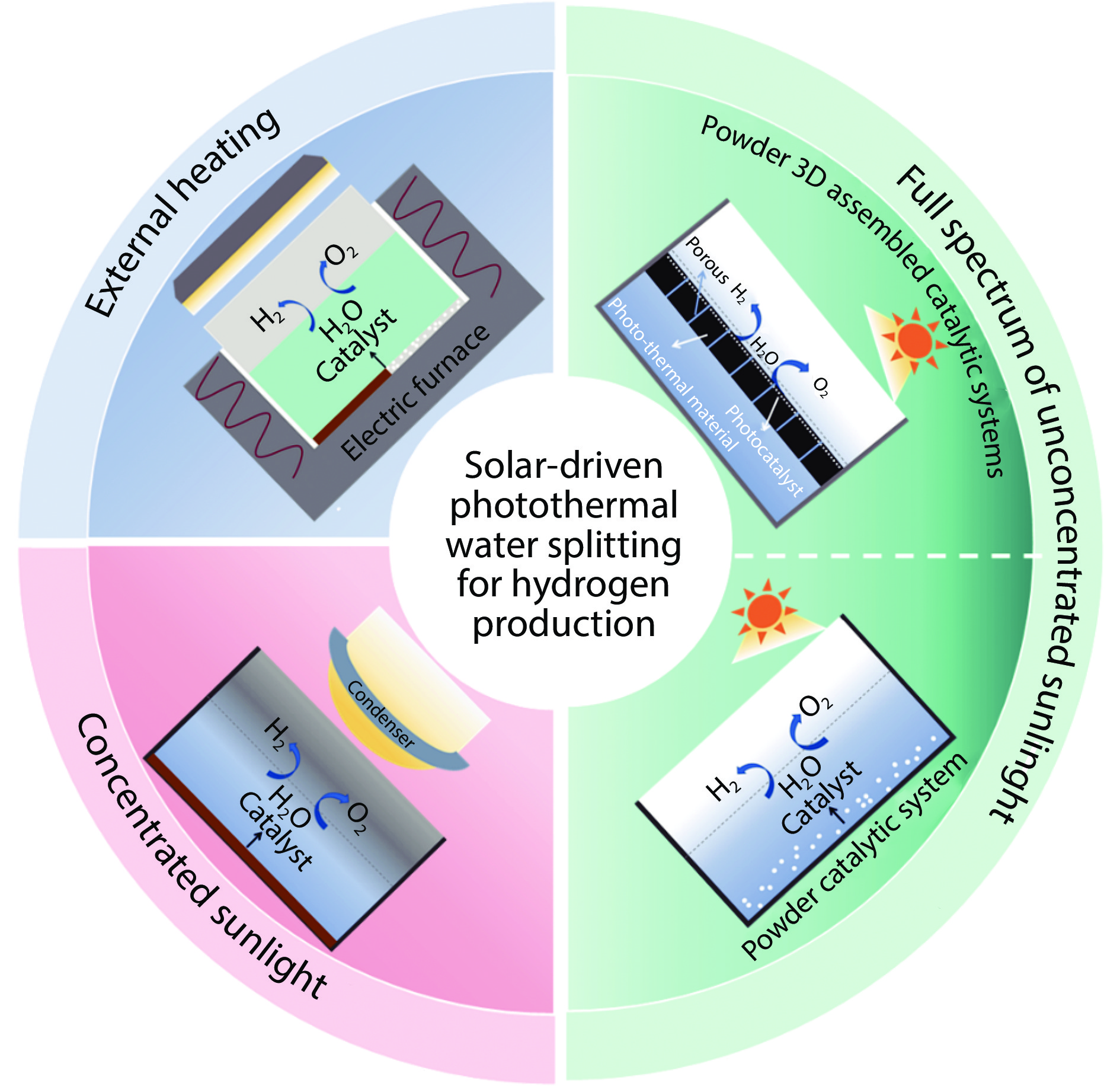
 DownLoad:
DownLoad: