| Citation: | Chunyan Qin, Zhilian Yue, Nieves Casañ-Pastor, Jun Chen, Gordon Wallace. The promises and future directions of wireless stimulation in biomedical applications[J]. Materials Lab, 2023, 2(2): 220058. doi: 10.54227/mlab.20220058 |
The promises and future directions of wireless stimulation in biomedical applications
-
Abstract
Wireless stimulation (WS) technologies have been developed as powerful strategies to modulate cellular behaviour and biological activity remotely and noninvasively through wireless manipulation of electrical signal. These WS systems are constructed from the electrically stimulus-responsive materials (magnetoelectric, piezoelectric, optoelectronic, and bipolar electroactive materials) that are triggered by the primary driving force, general like magnetic field, ultrasound, light, and electric field. With a deeper understanding of the integral role of electrical stimulation played in biological cells, tissues, and organs, WS has become the promising technique to work on neural cell stimulation, for either functional or repair effects, and other biological activities including drug release, electroporation and cancer treatment. This paper summarises existing WS systems in accordance with the utilised stimulus-responsive materials. Also, future directions of WS in potential biomedical applications are discussed. Along with the development of emerging techniques such as bipolar electrochemistry and 3D printing, more effective WS systems will be allowed to apply in biosystems with a change of paradigm.
-
-
References
1. R. Guduru, P. Liang, J. Hong, A. Rodzinski, A. Hadjikhani, J. Horstmyer, E. Levister, S. Khizroev, Nanomedicine, 2015, 10, 2051 2. G. Ciofani, S. Danti, D. D’Alessandro, L. Ricotti, S. Moscato, G. Bertoni, A. Falqui, S. Berrettini, M. Petrini, V. Mattoli, A. Menciassi, ACS Nano, 2010, 4, 6267 3. Y. S. Hsiao, Y. H. Liao, H. L. Chen, P. Chen, F. C. Chen, ACS Appl. Mater. Interfaces, 2016, 8, 9275 4. C. Qin, Z. Yue, Y. Chao, R. J. Forster, F. Maolmhuaidh, X. F. Huang, S. Beirne, G. G. Wallace, J. Chen, Appl. Mater. Today, 2020, 21, 100804 5. A. Singer, S. Dutta, E. Lewis, Z. Chen, J. C. Chen, N. Verma, B. Avants, A. K. Feldman, J. O’Malley, M. Beierlein, C. Kemere, J. T. Robinson, Neuron, 2020, 107, 631 6. J. Hopkins, L. Travaglini, A. Lauto, T. Cramer, B. Fraboni, J. Seidel, D. Mawad, Adv. Mater. Technol., 2019, 4, 1 7. N. A. Repina, A. Rosenbloom, A. Mukherjee, D. V. Schaffer, R. S. Kane, Annu. Rev. Chem. Biomol. Eng., 2017, 8, 13 8. J. Koo, M. R. MacEwan, S. K. Kang, S. M. Won, M. Stephen, P. Gamble, Z. Xie, Y. Yan, Y. Y. Chen, J. Shin, N. Birenbaum, S. Chung, S. B. Kim, J. Khalifeh, D. V. Harburg, K. Bean, M. Paskett, J. Kim, Z. S. Zohny, S. M. Lee, R. Zhang, K. Luo, B. Ji, A. Banks, H. M. Lee, Y. Huang, W. Z. Ray, J. A. Rogers, Nat. Med., 2018, 24, 1830 9. X. Wang, J. Law, M. Luo, Z. Gong, J. Yu, W. Tang, Z. Zhang, X. Mei, Z. Huang, L. You, Y. Sun, ACS Nano, 2020, 14, 3805 10. H. Wang, A. J. F. Tampio, Y. Xu, B. D. Nicholas, D. Ren, ACS Biomater. Sci. Eng., 2020, 6, 727 11. A. Marino, G. G. Genchi, M. Pisano, P. Massobrio, M. Tedesco, S. Martinoia, R. Raiteri, G. Ciofani, Neural Interface Engineering, 2020, 347 12. J. Kubanek, P. Shukla, A. Das, S. A. Baccus, M. B. Goodman, Journal of Neuroscience, 2018, 38, 3081 13. D. K. Piech, B. C. Johnson, K. Shen, M. M. Ghanbari, K. Y. Li, R. M. Neely, J. E. Kay, J. M. Carmena, M. M. Maharbiz, R. Muller, Nat. Biomed. Eng., 2020, 4, 207 14. A. Marino, S. Arai, Y. Hou, E. Sinibaldi, M. Pellegrino, Y. Chang, ACS Nano, 2015, 9, 7678 15. A. Marino, J. Barsotti, G. De Vito, C. Filippeschi, B. Mazzolai, V. Piazza, M. Labardi, V. Mattoli, G. Ciofani, ACS Appl. Mater. Interfaces, 2015, 7, 25574 16. M. Hoop, X. Z. Chen, A. Ferrari, F. Mushtaq, G. Ghazaryan, T. Tervoort, D. Poulikakos, B. Nelson, S. Pané, Sci. Rep., 2017, 7, 1 17. G. G. Genchi, L. Ceseracciu, A. Marino, M. Labardi, S. Marras, F. Pignatelli, L. Bruschini, V. Mattoli, G. Ciofani, Adv. Healthc. Mater., 2016, 5, 1808 18. G. G. Genchi, E. Sinibaldi, L. Ceseracciu, M. Labardi, A. Marino, S. Marras, G. De Simoni, V. Mattoli, G. Ciofani, Nanomedicine, 2018, 14, 2421 19. J. Li, K. Pu, Chem. Soc. Rev., 2019, 48, 38 20. R. Qazi, A. M. Gomez, D. C. Castro, Z. Zou, J. Y. Sim, Y. Xiong, J. Abdo, C. Y. Kim, A. Anderson, F. Lohner, S. H. Byun, B. Chul Lee, K. I. Jang, J. Xiao, M. R. Bruchas, J. W. Jeong, Nat. Biomed. Eng., 2019, 3, 655 21. T. C. Pappas, W. M. S. Wickramanyake, E. Jan, M. Motamedi, M. Brodwick, N. A. Kotov, Nano Lett., 2007, 7, 513 22. L. Bareket-Keren, Y. Hanein, Int. J. Nanomedicine, 2014, 9, 65 23. J. Li, J. Liu, C. Chen, ACS Nano, 2017, 11, 2403 24. Y. Wang, K. Xie, H. Yue, X. Chen, X. Luo, Q. Liao, M. Liu, F. Wang, P. Shi, Nanoscale, 2020, 12, 2406 25. J. G. McCall, T. Il Kim, G. Shin, X. Huang, Y. H. Jung, R. Al-Hasani, F. G. Omenetto, M. R. Bruchas, J. A. Rogers, Nat. Protoc., 2013, 8, 2413 26. S. Löffler, B. Libberton, A. Richter-Dahlfors, Electronics (Switzerland), 2015, 4, 879 27. M. Jakešová, M. Silverå Ejneby, V. Đerek, T. Schmidt, M. Gryszel, J. Brask, R. Schindl, D. T. Simon, M. Berggren, F. Elinder, E. D. Głowacki, Sci. Adv., 2019, 5, eaav5265 28. Y. Wu, Y. Peng, H. Bohra, J. Zou, V. D. Ranjan, Y. Zhang, Q. Zhang, M. Wang, ACS Appl. Mater. Interfaces, 2019, 11, 4833 29. H. Sun, D. Yu, Y. Guan, Z. Du, J. Ren, X. Qu, Chemical Communications, 2019, 55, 9833 30. W. Li, R. Luo, X. Lin, A. D. Jadhav, Z. Zhang, L. Yan, C. Y. Chan, X. Chen, J. He, C. H. Chen, P. Shi, Biomaterials, 2015, 65, 76 31. C. Qin, Z. Yue, X. F. Huang, R. J. Forster, G. G. Wallace, J. Chen, Appl. Mater. Today, 2022, 27, 101481 32. C. Qin, Z. Yue, X.-F. Huang, R. J. Forster, G. G. Wallace, J. Chen, Data Brief, 2022, 43, 108393 33. C. Qin, Z. Yue, Y. Chao, R. J. Forster, F. Maolmhuaidh, X. F. Huang, S. Beirne, G. G. Wallace, J. Chen, Data Brief, 2020, 33, 106406 34. N. Shida, Y. Zhou, S. Inagi, Acc. Chem. Res., 2019, 52, 2598 35. S. E. Fosdick, K. N. Knust, K. Scida, R. M. Crooks, Angewandte Chemie International Edition, 2013, 52, 10438 36. R. M. Crooks, ChemElectroChem, 2016, 3, 357 37. A. M. Rajnicek, Z. Zhao, J. Moral-Vico, A. M. Cruz, C. D. McCaig, N. Casañ-Pastor, Adv. Healthc. Mater., 2018, 7, 1800473 38. J.-C. Bradley, H.-M. Chen, J. Crawford, J. Eckert, K. Ernazarova, T. Kurzeja, M. Lin, M. McGee, W. Nadler, S. G. Stephens, Nature, 1997, 389, 268 39. J. Bradley, J. Crawford, K. Ernazarova, M. McGee, S. G. Stephens, Advanced Materials, 1997, 9, 1168 40. L. Fuentes-Rodriguez, L. Abad, L. Simonelli, D. Tonti, N. Casañ-Pastor, Journal of Physical Chemistry C, 2021, 125, 16629 41. Y. Yang, C. Wang, S. Ashraf, G. G. Wallace, RSC Adv., 2013, 3, 5447 42. R. Guo, J. N. Barisci, P. C. Innis, C. O. Too, G. G. Wallace, D. Zhou, Synth. Met., 2000, 114, 267 43. X. Liu, K. J. Gilmore, S. E. Moulton, G. G. Wallace, J. Neural Eng., 2009, 6, 065002 44. K. J. Gilmore, M. Kita, Y. Han, A. Gelmi, M. J. Higgins, S. E. Moulton, G. M. Clark, R. Kapsa, G. G. Wallace, Biomaterials, 2009, 30, 5292 45. J. U. Khan, A. Ruland, S. Sayyar, B. Paull, J. Chen, P. C. Innis, Lab Chip, 2021, 21, 3979 46. R. K. Perdue, D. R. Laws, D. Hlushkou, U. Tallarek, R. M. Crooks, Anal. Chem., 2009, 81, 10149 47. L. Chen, A. Ghiasvand, S. C. Lam, E. S. Rodriguez, P. C. Innis, B. Paull, Anal. Chim. Acta, 2022, 1193, 338810 48. D. R. Laws, D. Hlushkou, R. K. Perdue, U. Tallarek, R. M. Crooks, Anal. Chem., 2009, 81, 8923 49. R. Chen, G. Romero, M. G. Christiansen, A. Mohr, P. Anikeeva, Science, 2015, 347, 1477 50. A. M. Lozano, N. Lipsman, H. Bergman, P. Brown, S. Chabardes, J. W. Chang, K. Matthews, C. C. McIntyre, T. E. Schlaepfer, M. Schulder, Y. Temel, J. Volkmann, J. K. Krauss, Nat. Rev. Neurol., 2019, 15, 148 51. C. Qin, Z. Yue, G. G. Wallace, J. Chen, ACS Appl. Bio. Mater., 2022, 5, 5041 52. J. Moral-Vico, N. M. Carretero, E. Pérez, C. Suñol, M. Lichtenstein, N. Casañ-Pastor, Electrochim. Acta, 2013, 111, 250 53. B. C. Thompson, J. Chen, S. E. Moulton, G. G. Wallace, Nanoscale, 2010, 2, 499 54. A. J. Evans, B. C. Thompson, G. G. Wallace, R. Millard, S. J. O’Leary, G. M. Clark, R. K. Shepherd, R. T. Richardson, J. Biomed. Mater. Res. A, 2009, 91, 241 55. R. T. Richardson, B. Thompson, S. Moulton, C. Newbold, M. G. Lum, A. Cameron, G. Wallace, R. Kapsa, G. Clark, S. O’Leary, Biomaterials, 2007, 28, 513 56. B. C. Thompson, R. T. Richardson, S. E. Moulton, A. J. Evans, S. O’Leary, G. M. Clark, G. G. Wallace, Journal of Controlled Release, 2010, 141, 161 57. C. D. O’Connell, S. Konate, C. Onofrillo, R. Kapsa, C. Baker, S. Duchi, T. Eekel, Z. Yue, S. Beirne, G. Barnsley, C. Di Bella, P. F. Choong, G. G. Wallace, Bioprinting, 2020, 19, e00087 58. Y. Fan, Z. Yue, E. Lucarelli, G. G. Wallace, Adv. Healthc. Mater., 2020, 9, 1 -
Rights and permissions
This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.
Information
Article Metrics
-
Figure 1.
Bipolar electroactive conjugated polymers based WS systems. Bipolar electrochemical activity of PPy was realised and enhanced with PMAS incorporation in biological solution, displaying gradient colours across the surfaces (left). Soft PPy matrix was obtained with removal of rigid substrate (middle). Both rat pheochromocytoma cell (PC 12) and human neuroblastoma cell (SH-SY5Y) differentiation was promoted due to the enhanced bipolar electroactivity and the addition of softness property (right).[4,31–33] Copyright 2020, 2022 Elsevier Ltd.
-
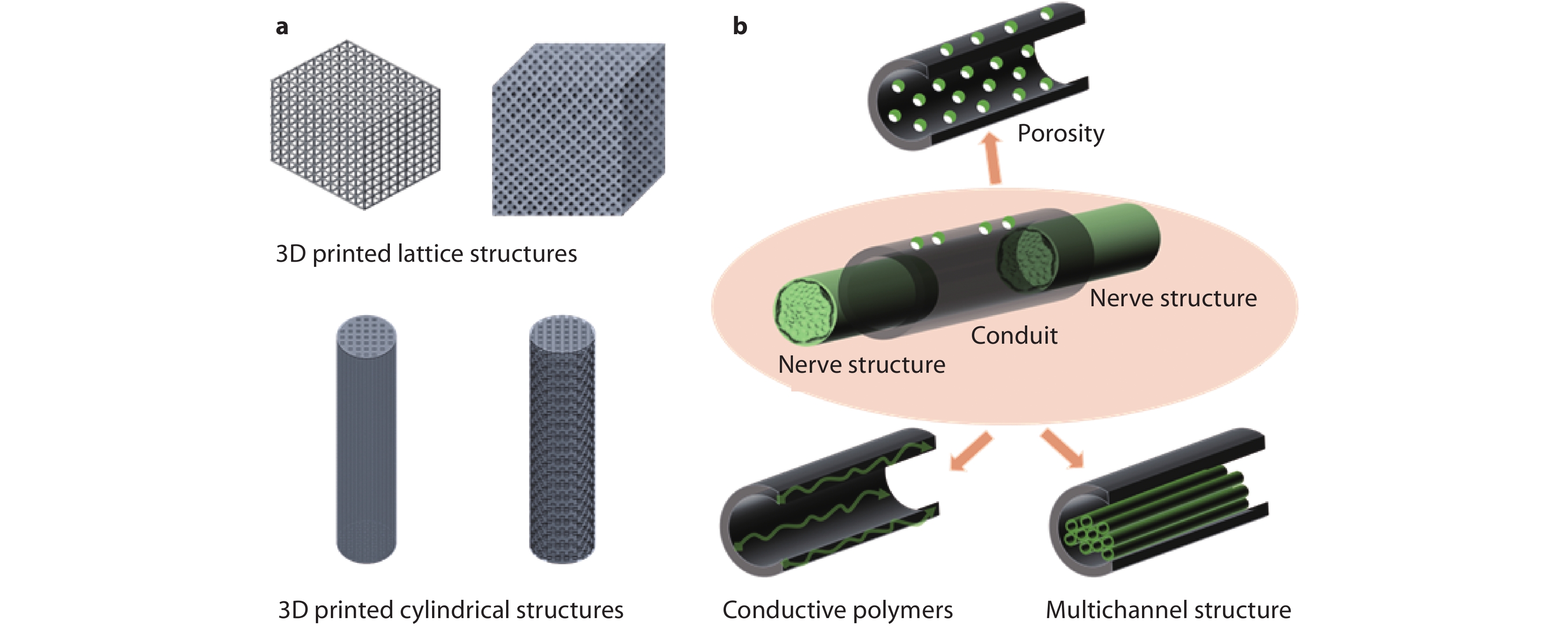
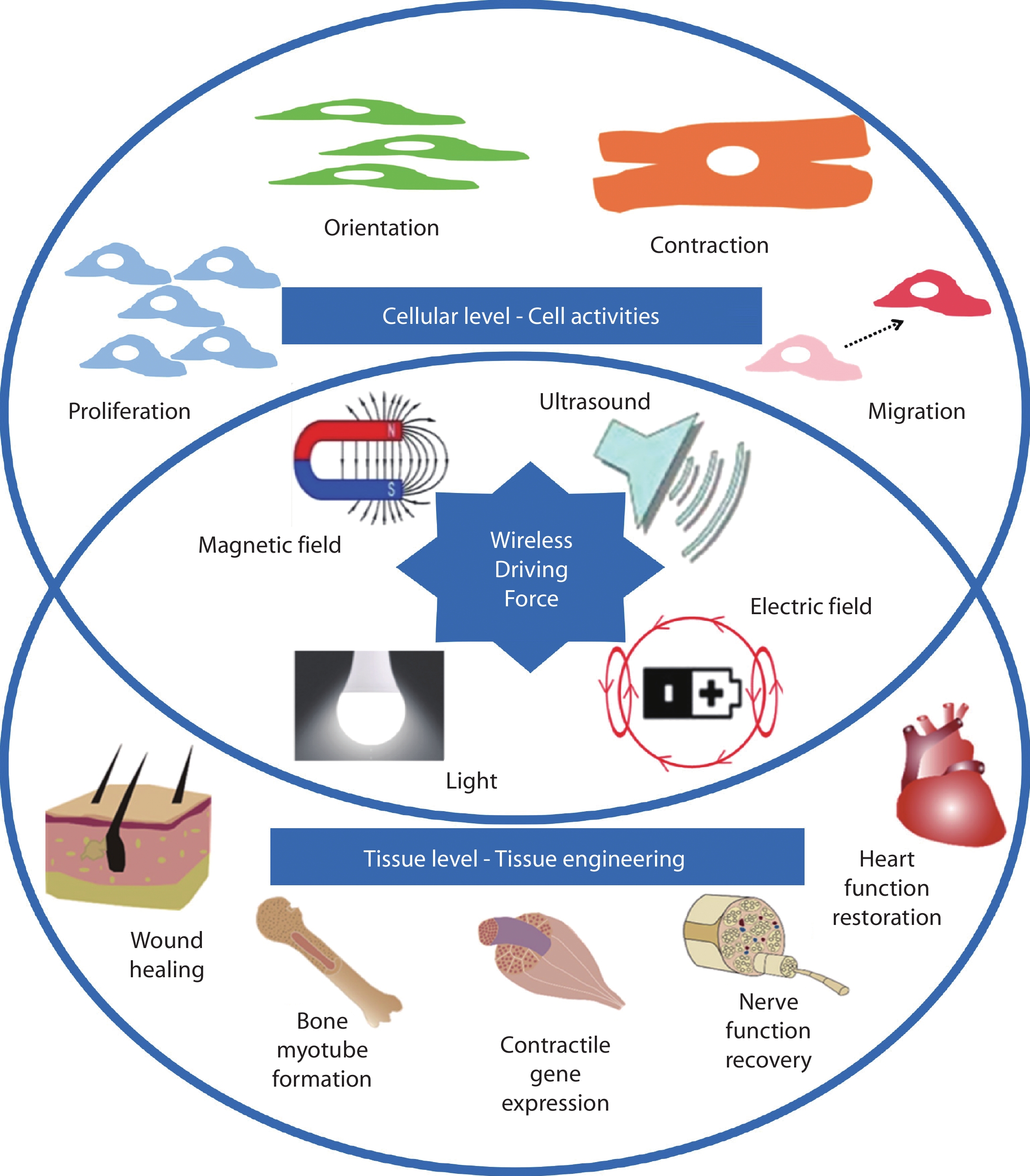
Figure 2.
Schematic of various 3D printed bipolar electroactive architectures. a Lattice and cylindrical structures and b nerve conduits.

 Chunyan Qin is currently a postdoctoral research fellow at Intelligent Polymer Research Institute (IPRI), University of Wollongong (UOW). She received her PhD degree there in 2022. Her research interests lie in biomaterials, bio interfaces, cell culture/stimulation, drug delivery, biosensors, biomedical devices etc. In 2019, she received the Bill Wheeler Award for best communicating the social impact of her bionics research.
Chunyan Qin is currently a postdoctoral research fellow at Intelligent Polymer Research Institute (IPRI), University of Wollongong (UOW). She received her PhD degree there in 2022. Her research interests lie in biomaterials, bio interfaces, cell culture/stimulation, drug delivery, biosensors, biomedical devices etc. In 2019, she received the Bill Wheeler Award for best communicating the social impact of her bionics research.  ZhilianYue is currently a principle research fellow in the intelligent Polymer Research Institute, University of Wollongong. Her research interests include tissue engineering and regenerative medicine, 3D bioprinting and medical bionics.
ZhilianYue is currently a principle research fellow in the intelligent Polymer Research Institute, University of Wollongong. Her research interests include tissue engineering and regenerative medicine, 3D bioprinting and medical bionics.  Nieves Casañ-Pastor is Research Professor at teh CSIC Institut de Ciencia de Materials de Barcelona, Spain. Her reserach interests are electroactive materials with mixed valence and mixed conductivity as electrodes, and the local resolution required to characterize gradient materials, as well as the implications in biostimulation and energy storage. She has been CSIC Scientific Comitte a¡Advisor and has recieved a number of Grants and Awards.
Nieves Casañ-Pastor is Research Professor at teh CSIC Institut de Ciencia de Materials de Barcelona, Spain. Her reserach interests are electroactive materials with mixed valence and mixed conductivity as electrodes, and the local resolution required to characterize gradient materials, as well as the implications in biostimulation and energy storage. She has been CSIC Scientific Comitte a¡Advisor and has recieved a number of Grants and Awards.  Jun Chen is currently appointed as Associate Dean of Australian Institute for Innovative Materials (AIIM), and Head of Postgraduate Studies of IPRI/UOW. His research interests include electroactive materials, catalysis, sustainable energy devices/systems, electro-/bio-interfaces, nano/micro- materials, 2D/3D printing and wearable electronic devices. He has authored over 260 peer-reviewed publications in international journals with an h-index of 78. Chen has been identified as Highly Cited Researchers in Cross Field (2018|2020-2022) and received Vice-Chancellor's Award for Researcher of the Year (UOW) in 2021.
Jun Chen is currently appointed as Associate Dean of Australian Institute for Innovative Materials (AIIM), and Head of Postgraduate Studies of IPRI/UOW. His research interests include electroactive materials, catalysis, sustainable energy devices/systems, electro-/bio-interfaces, nano/micro- materials, 2D/3D printing and wearable electronic devices. He has authored over 260 peer-reviewed publications in international journals with an h-index of 78. Chen has been identified as Highly Cited Researchers in Cross Field (2018|2020-2022) and received Vice-Chancellor's Award for Researcher of the Year (UOW) in 2021.  Gordon G. Wallace is director of IPRI, ACES, and ANFF (Materials Node). He leads a world-class integrated multidisciplinary and multi-organisational team with a global impact in the design and utilisation of electromaterials for bionics. His research has resulted in excess of 1,020 refereed publications, with more than 50,000 citations, and an h-index of 103. The quality of CI Wallace’s work is attested by the June 2018 data from the Clarivate Analytics’ Essential Science Indicators lists, which rank the top 1% of authors of papers and 1% of papers by citations, published in the last 10 years.
Gordon G. Wallace is director of IPRI, ACES, and ANFF (Materials Node). He leads a world-class integrated multidisciplinary and multi-organisational team with a global impact in the design and utilisation of electromaterials for bionics. His research has resulted in excess of 1,020 refereed publications, with more than 50,000 citations, and an h-index of 103. The quality of CI Wallace’s work is attested by the June 2018 data from the Clarivate Analytics’ Essential Science Indicators lists, which rank the top 1% of authors of papers and 1% of papers by citations, published in the last 10 years. 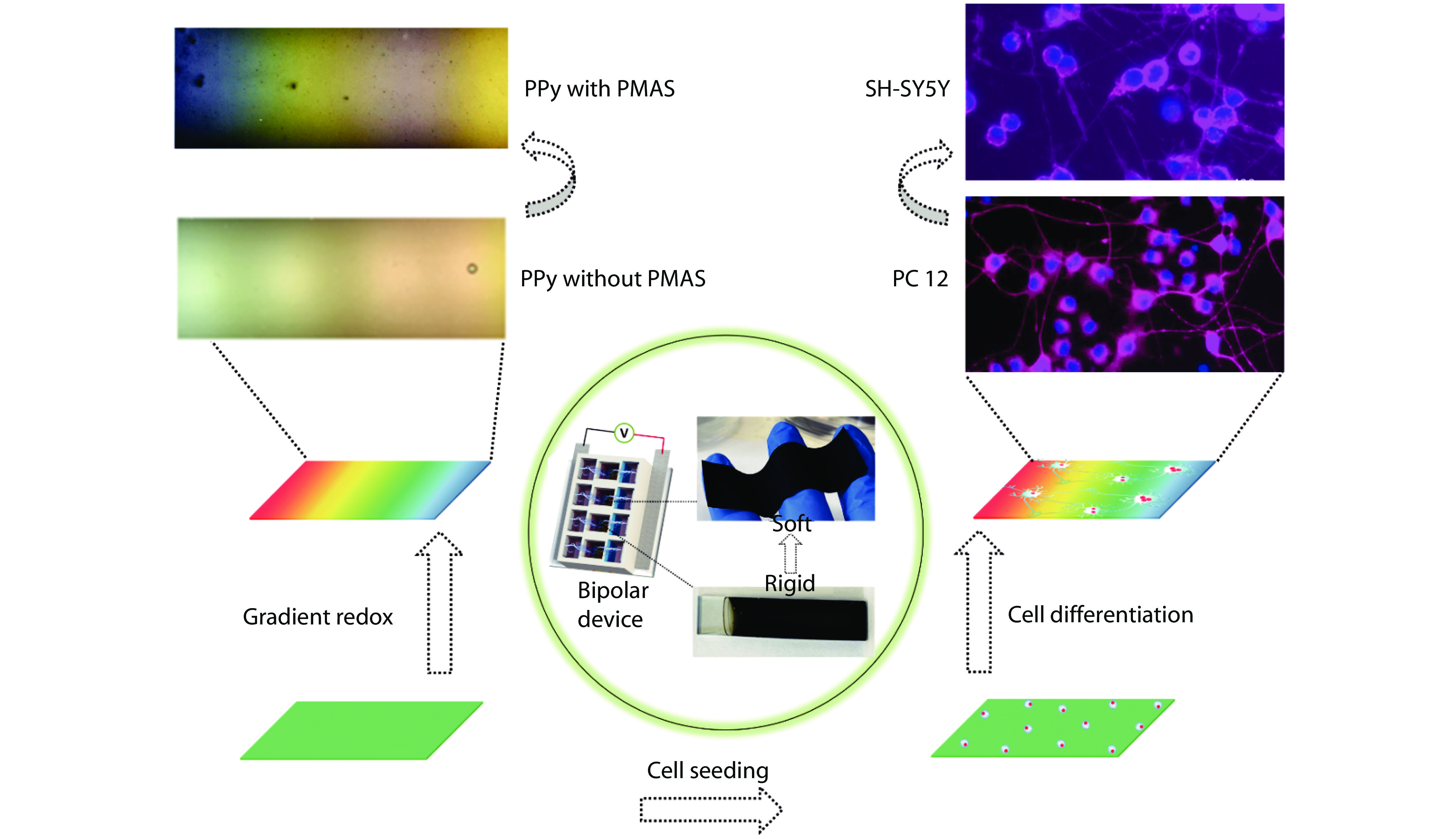
 DownLoad:
DownLoad: