| Citation: | Ju Li, Renzhe Jin, Mengyuan He, Qiang Zhao, Zhengyu Wei, Panfei Sun, Dehai Ping, Xiaomei Yu, Songjie Li. Preparation and anti-corrosion properties of 8-HQ@SiO2@AK/EP composite materials[J]. Materials Lab, 2024, 3(1): 230018. doi: 10.54227/mlab.20230018 |
Preparation and anti-corrosion properties of 8-HQ@SiO2@AK/EP composite materials
-
Abstract
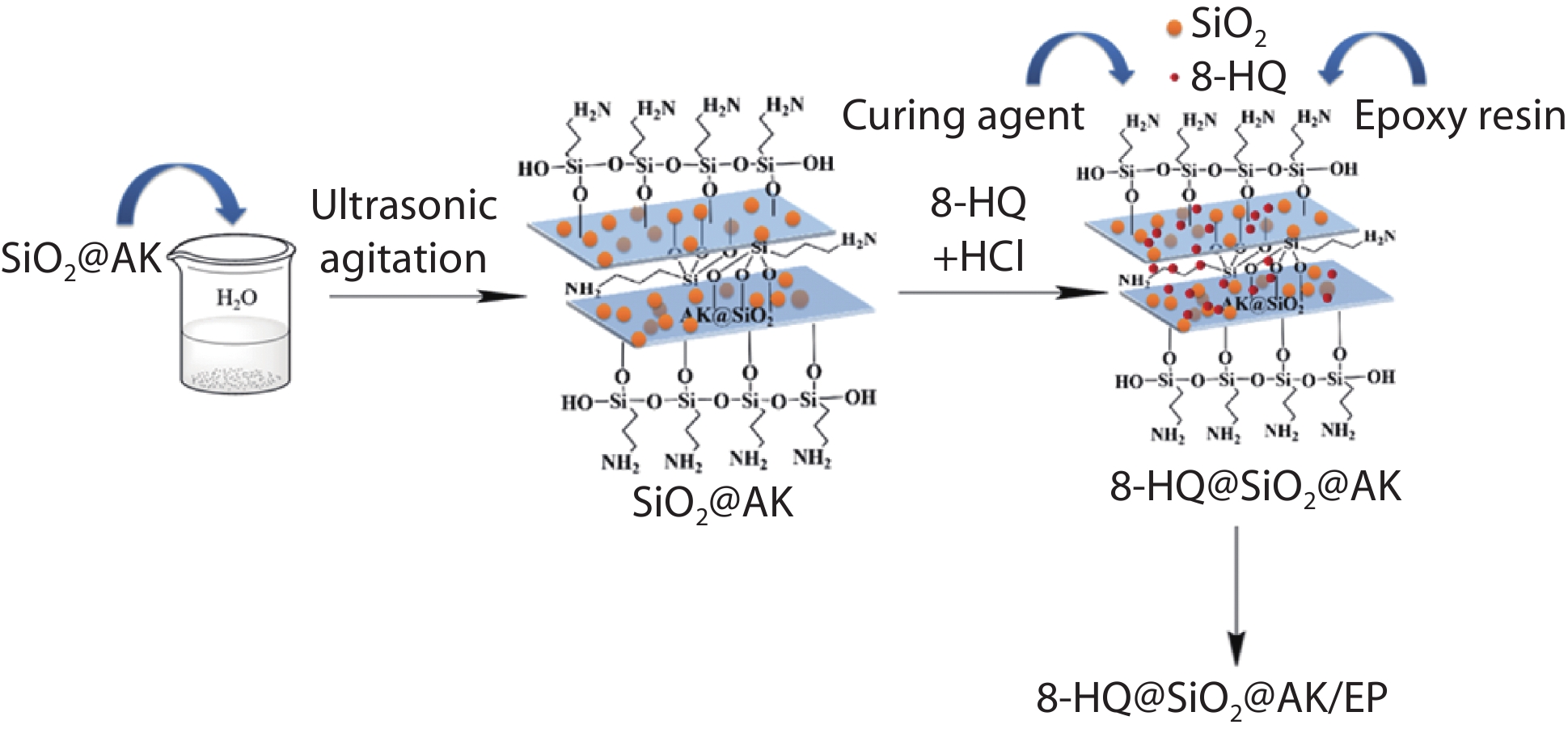
Epoxy coatings are popular due to their strong adhesion, low cost, and superior thermal and electrical insulation. Unfortunately, surface defects in epoxy resin lead to surface cracks, limiting the long-term use of epoxy resin coatings in humid marine environments. In this study, modified kaolin (Kaolin) was added to an epoxy resin (EP) to enhance its corrosion resistance, and a silane coupling agent 3-aminopropyltriethoxysilane (APTES), tetraethyl orthosilicate (TEOS), and 8-hydroxyquinoline (8-HQ) were chosen as modifiers. This resulted in the composite 8-hydroxyquinoline/silica/amino kaolin (8-HQ@SiO2@AK), and the structural makeup, loading, hydrophobic characteristics, surface roughness, and corrosion resistance of 8-HQ@SiO2@AK and 8-hydroxyquinoline/silica/amino kaolin/epoxy resin composite coating (8-HQ@SiO2@AK/EP) was investigated. Based on these findings, the maximum contact angle of the hydrophobic composite coating was 88°, its roughness Ra was 3.09 nm, and the hardness and adhesion were unaltered compared to silica-modified kaolin/epoxy composite coatings (SiO2@AK/EP). The impedance modulus |Z|0.01 Hz of the composite was 3.662×109 Ω • cm2 after 30 days of immersion, which indicated significantly enhanced corrosion resistance.
-
-
References
1. B. Ehsan, J. Ali, R. Zahra, S. Sarah, S. Mohammad Reza, Progress in Organic Coatings, 2014, 77, 1169 2. K. Chang, M. Hsu, H. Lu, M. Lai, P. Liu, C. Hsu, W. Ji, T. Chuang, Y. Wei, J. Yeh, W. Liu, Carbon, 2014, 66, 144 3. L. Neill, S. Gin, T. Ducasse, T. D. Echave, M. Fournier, P. Jollivet, A. Gourgiotis, N. A. Wall, NPJ Materials Degradation, 2017, 1, 1 4. I. Lanneluc, M. Langumier, R. Sabot, M. Jeannin, P. Refait, S. Sablé, International Biodeterioration & Biodegradation, 2015, 99, 55 5. S. K. Dhaliwal, B. P. Meek, M. M. Modirrousta, Frontiers in Psychiatry, 2015, 6, 7 6. B. Zhang, J. Li, X. Zhao, X. Hu, L. Yang, N. Wang, Y. Li, B. Hou, Chemical Engineering Journal, 2016, 306, 441 7. X. Wei, C. Ning, Z. Lu, J. Zhang, Ceramics International, 2022, 48, 8440 8. M. D. Maksimović, V. B. Mišković-Stanković, Corrosion Science, 1992, 33, 271 9. H. Bi, J. Sykes, Progress in Organic Coatings, 2015, 87, 83 10. M. Sababi, H. Terryn, J. M. C. Mol, Progress in Organic Coatings, 2017, 105, 29 11. M. M. Tabatabaei, D. Mahsa, R. Mohammad, G. Ebrahim, R. Bahram, M. Mohammad, Construction and Building Materials, 2020, 247, 118555 12. F. Meng, L. Liu, W. Tian, H. Wu, Y. Li, T. Zhang, F. Wang, Corrosion Science, 2015, 101, 139 13. R. Shamim, D. A. Asghar Sarabi, M. Javad, Applied Surface Science, 2018, 440, 880 14. M. Tamilselvi, P. Kamaraj, M. Arthanareeswari, S. Devikala, J. Selvi, Applied Surface Science, 2015, 332, 12 15. A. Ghanbari, M. M. Attar, Journal of Industrial & Engineering Chemistry, 2015, 23, 145 16. S. S. Golru, M. M. Attar, B. Ramezanzadeh, Progress in Organic Coatings, 2014, 77, 1391 17. L. Liu, J. Xu, Vacuum, 2011, 85, 687 18. B. Ramezanzadeh, M. M. Attar, M. Farzam, Progress in Organic Coatings, 2011, 72, 410 19. T. A. Truc, T. T. Thuy, V. K. Oanh, T. T. X. Hang, A. S. Nguyen, N. Causse, N. Pebere, Surfaces and Interfaces, 2019, 14, 26 20. K. Zhang, L. Wang, G. Liu, Corrosion Science, 2013, 75, 38 21. F. Sara, P. Massimo, P. Giovanni, N. T. Dintcheva, M. Pierluigi, Polymer Degradation and Stability, 2011, 96, 823 -
Rights and permissions
This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.
Information
Article Metrics
-
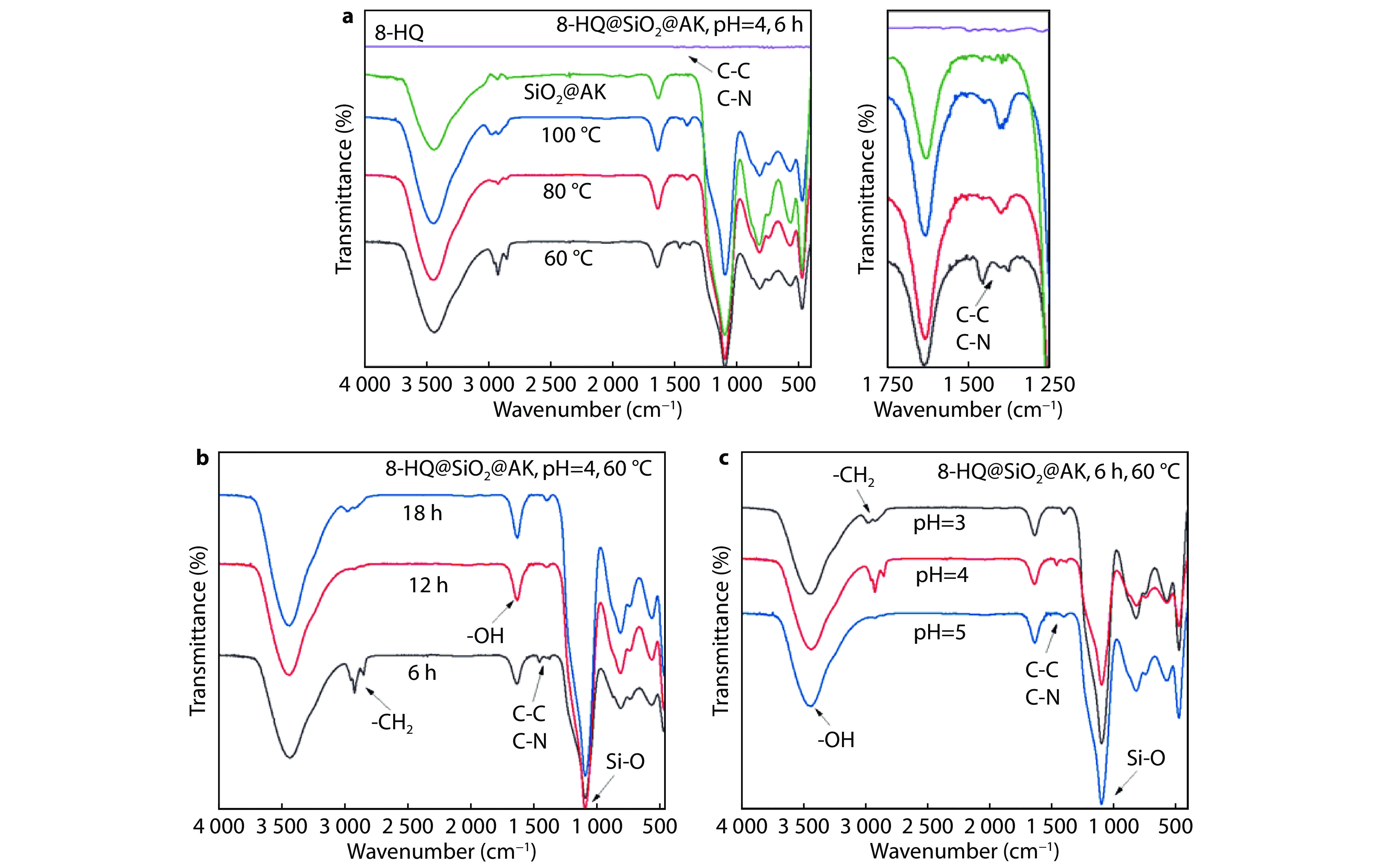
Figure 1.
FTIR diagrams of prepared 8-HQ @SiO2@AK in different conditions: a different reaction temperature; b different reactant time and c different reaction pH.
-
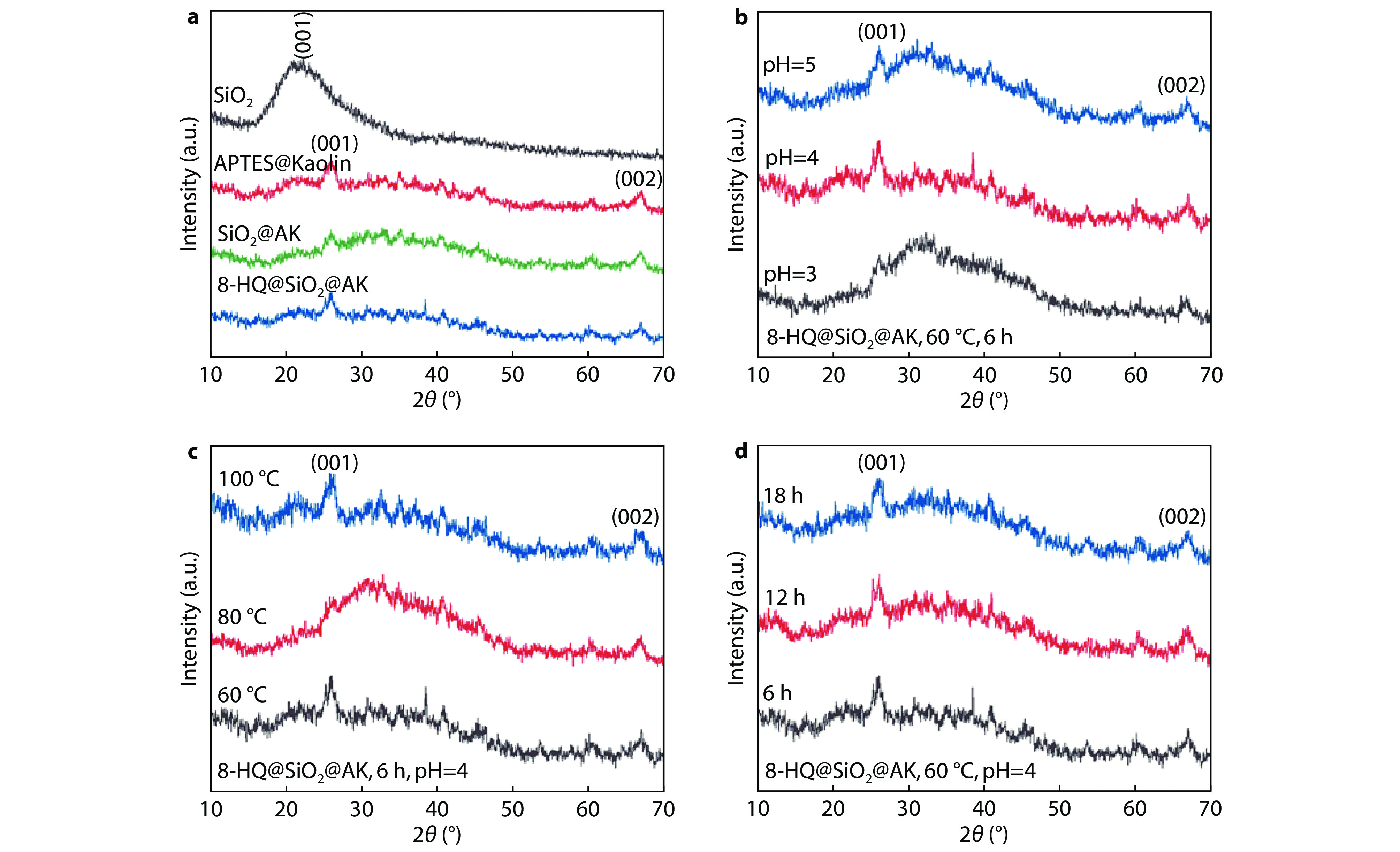
Figure 2.
XRD diagrams of 8-HQ@SiO2@AK prepared in different conditions: a comparison chart before and after modification; b different reaction pH; c different reaction time and d different reactant temperature.
-
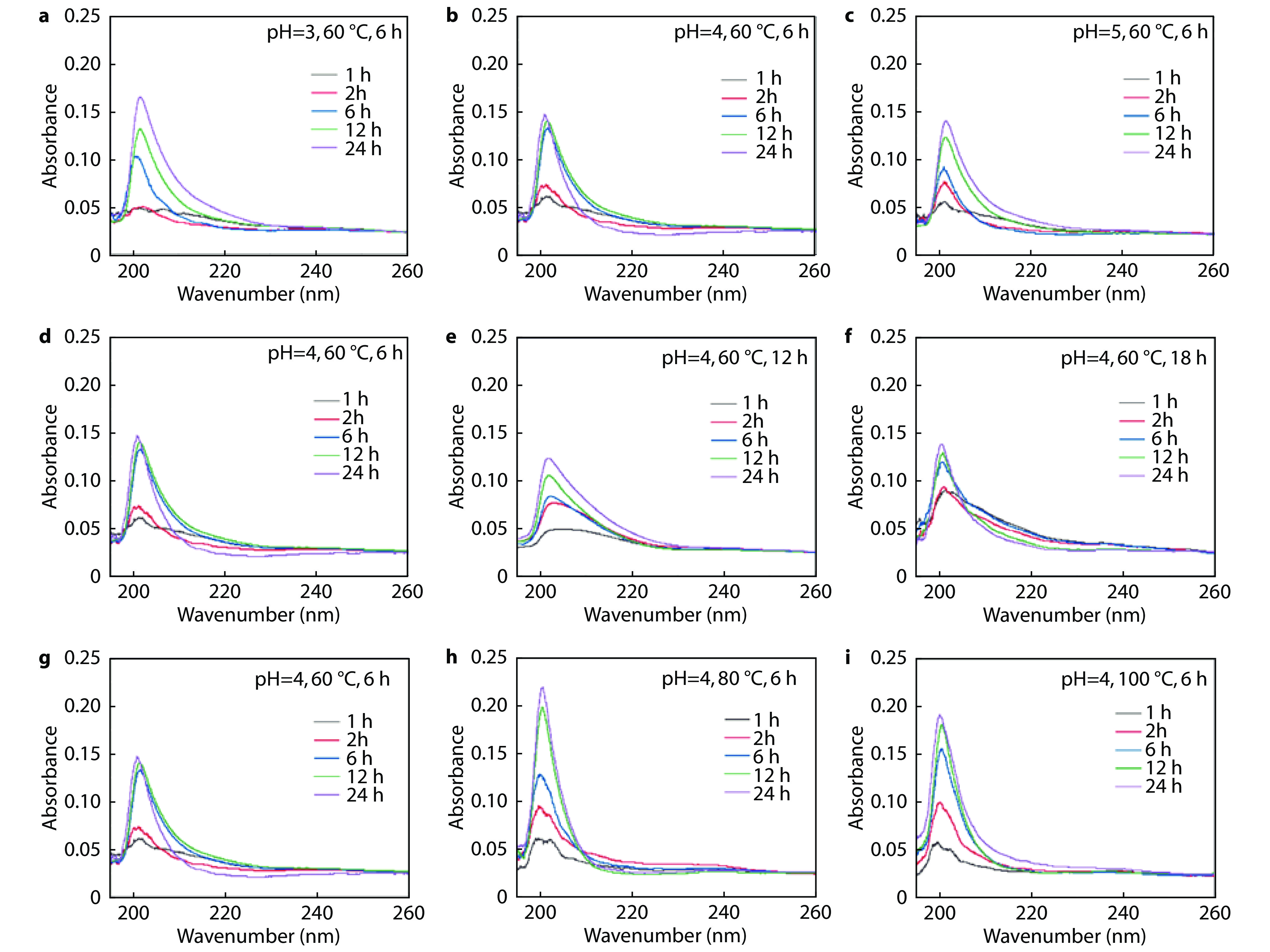
Figure 3.
UV diagrams of 8-HQ@SiO2@AK prepared in different conditions: a-c different reaction pH; d-f different reaction time and g-i different reaction temperature.
-
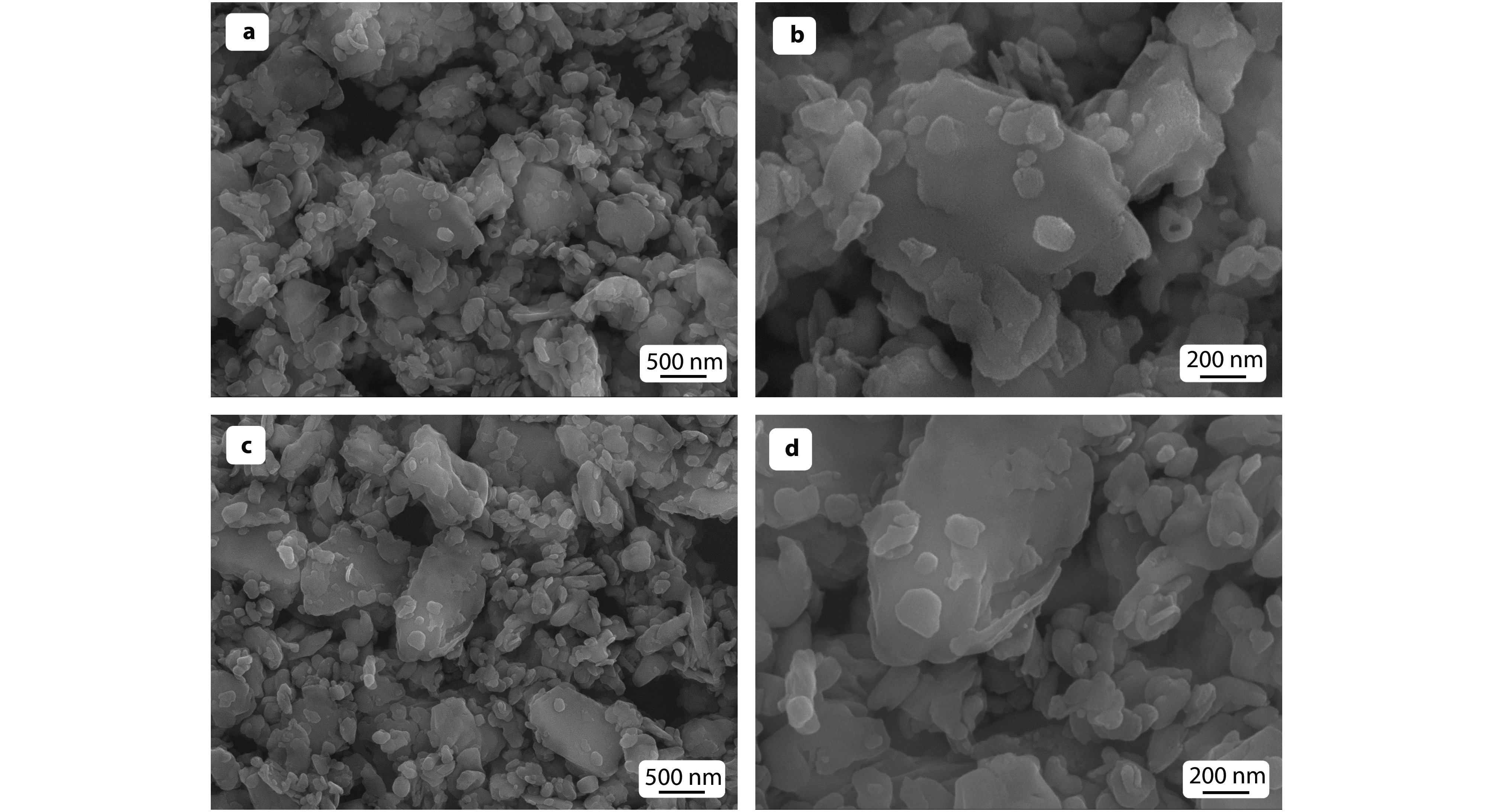
Figure 4.
SEM images of a-b SiO2@AK and c-d 8-HQ@SiO2@AK.
-
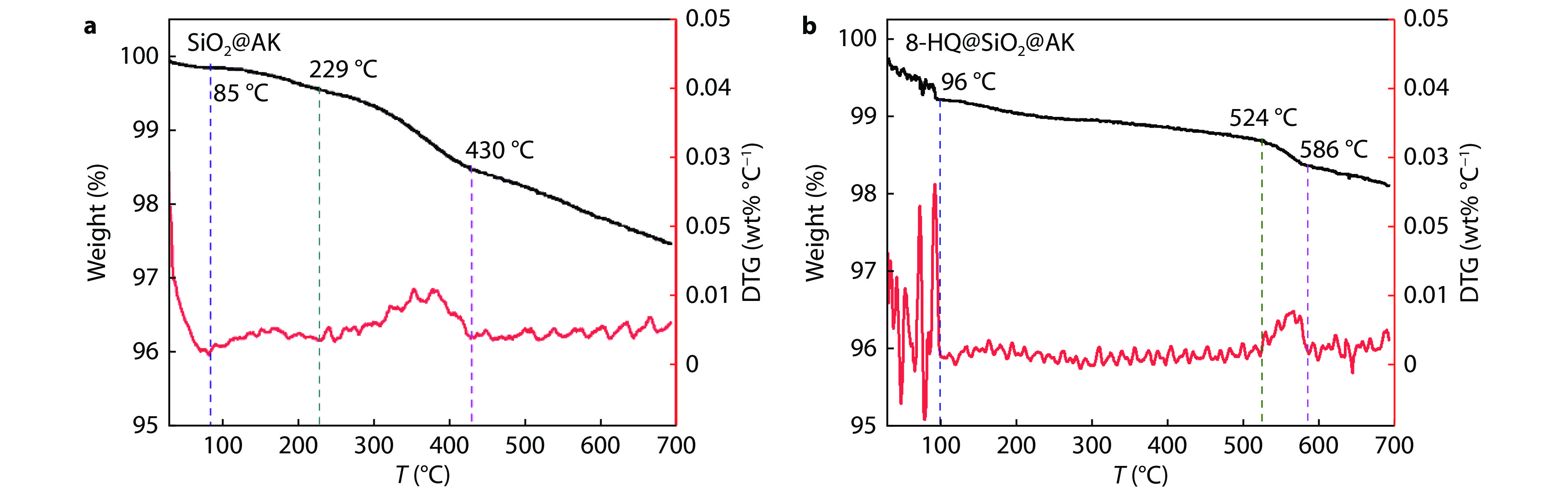
Figure 5.
TG and DTG curves of a SiO2@AKand b 8-HQ@SiO2@AK.
-
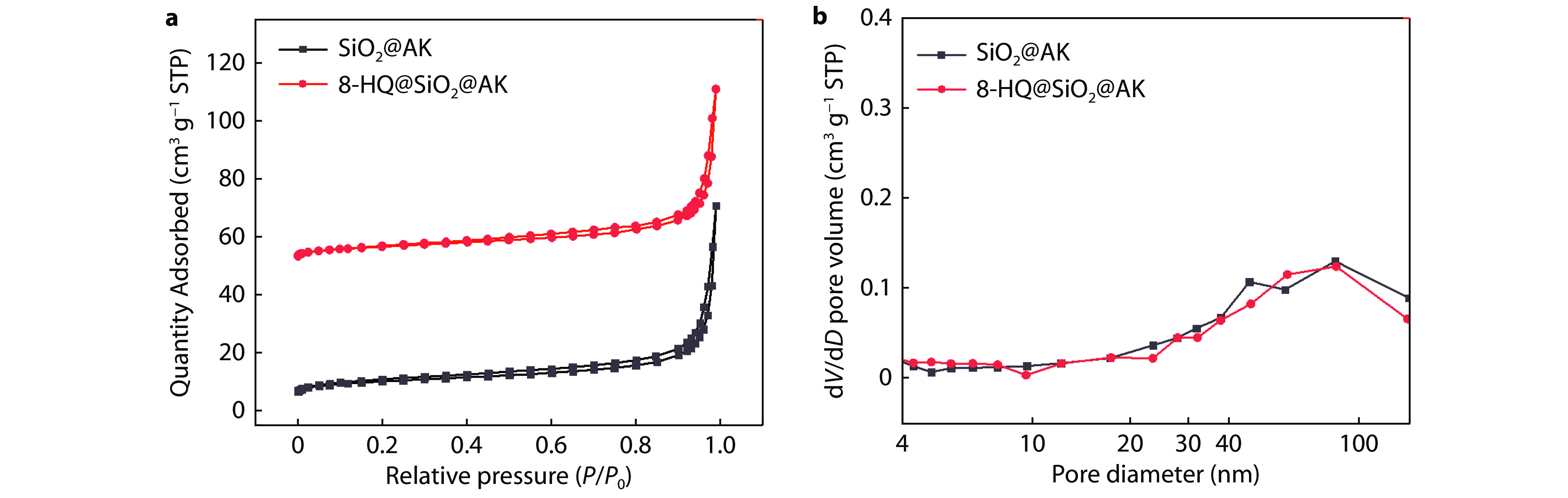
Figure 6.
N2 adsorption and desorption isotherm of a 8-HQ@SiO2@AK and b the corresponding pore size of materials.
-
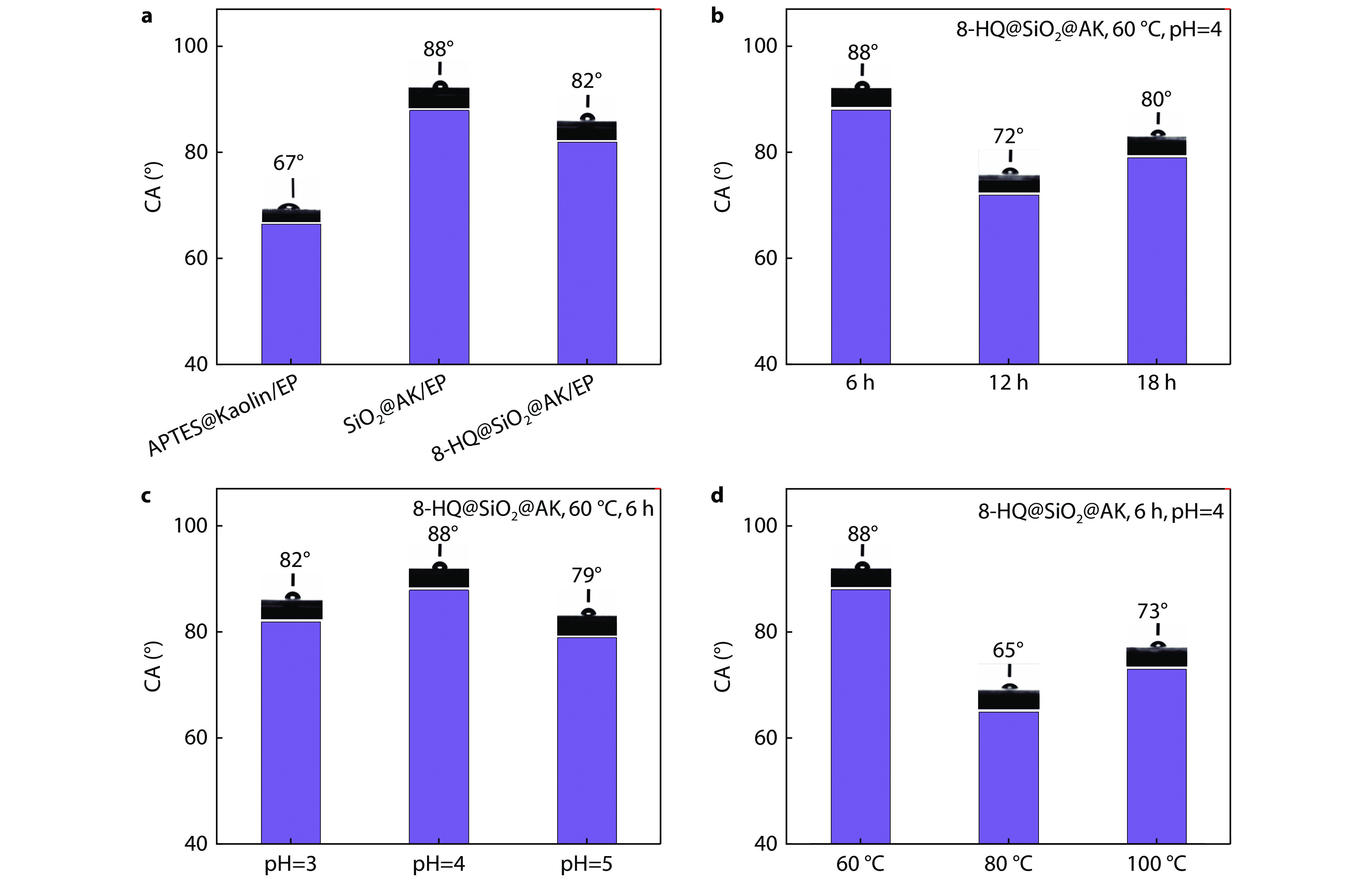
Figure 7.
Histograms of water contact angle test of SiO2@AK/EP coatings in different reaction conditions: a the water contact angle before and after modification of composite coatings; b different reaction time; c different reaction pH and d different reaction temperature.
-
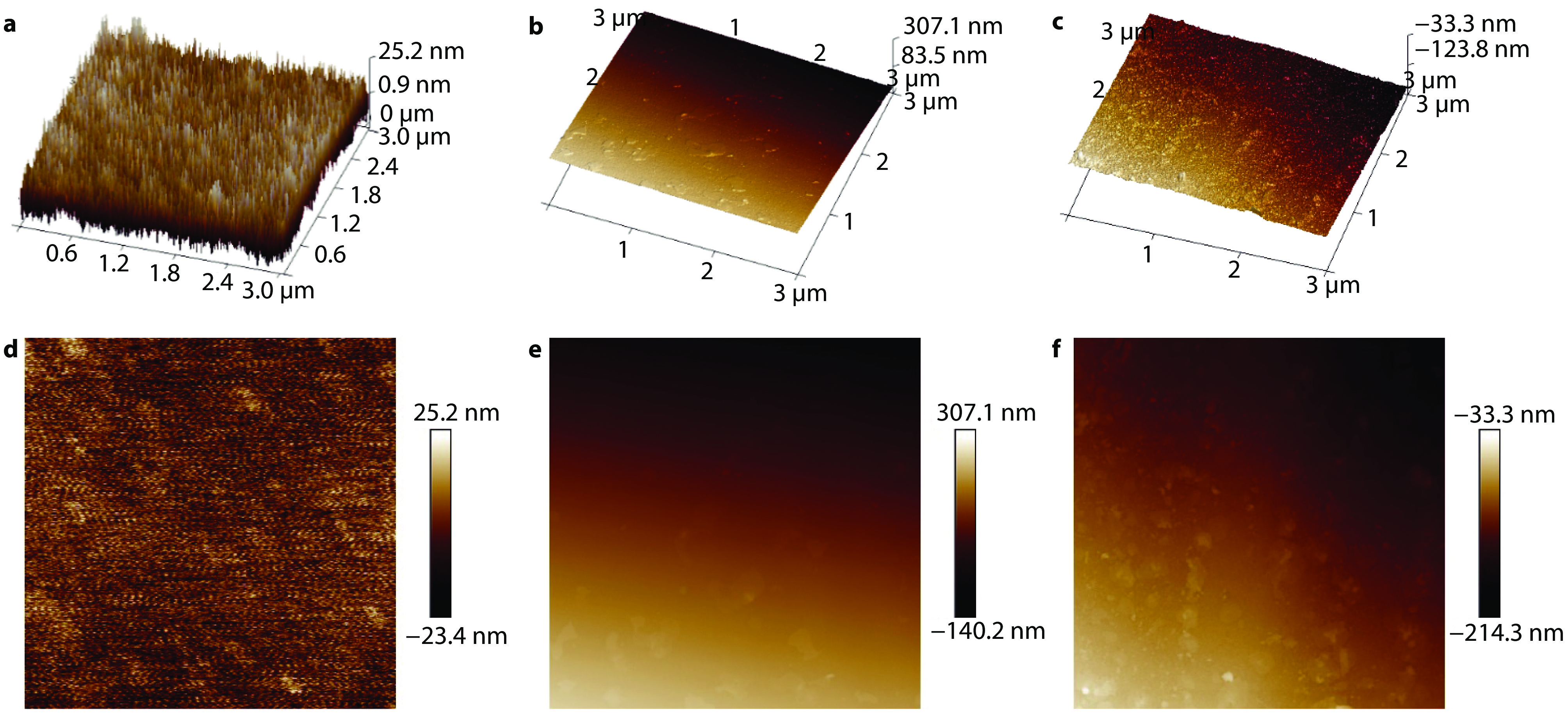
Figure 8.
The AFM images of a APTES@Kaolin/EP; b SiO2@AK/EP and c 8-HQ@SiO2@AK/EP coatings.
-
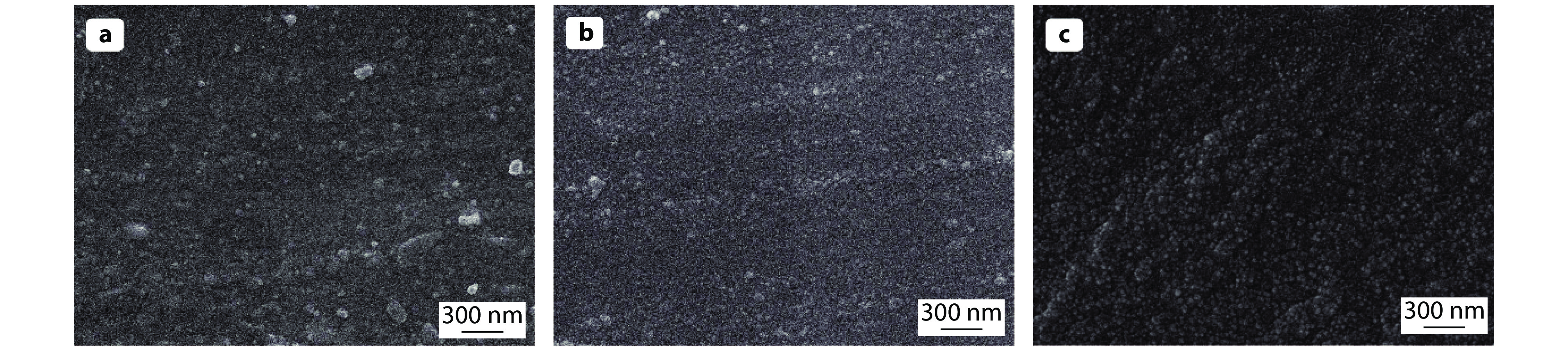
Figure 9.
The SEM images of a APTES@Kaolin/EP, b SiO2@AK/EP and c 8-HQ@SiO2@AK/EP coatings.
-
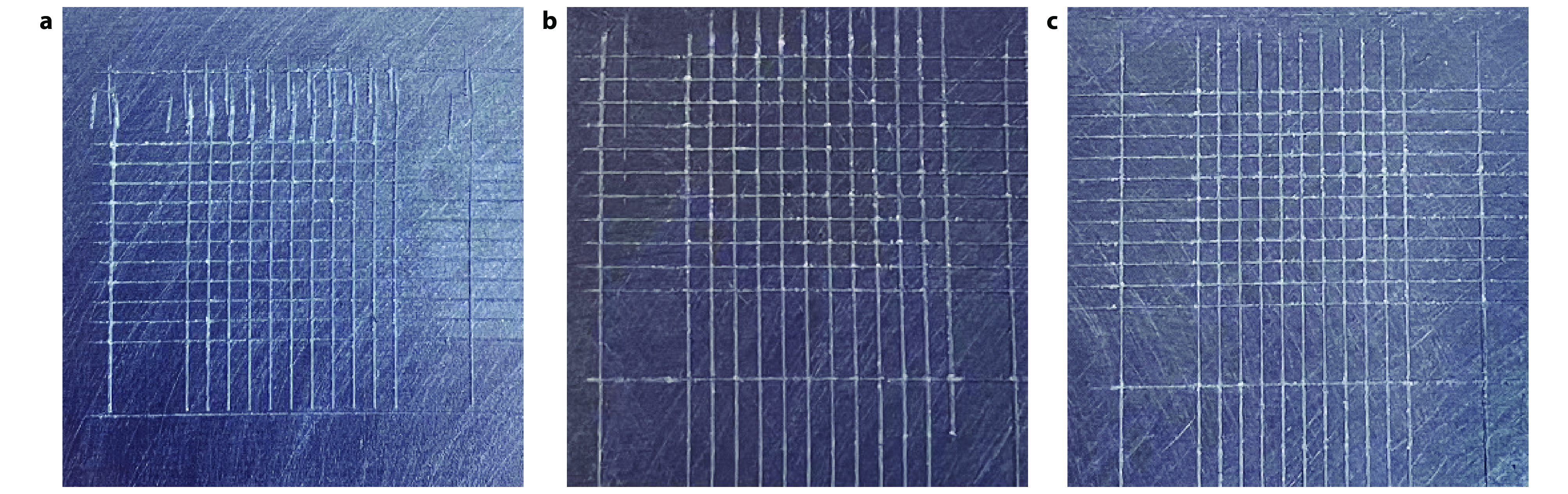
Figure 10.
Paint film cross-cut test pictures of a APTES@Kaolin/EP, b SiO2@AK/EP and c 8-HQ@SiO2@AK/EP coatings.
-
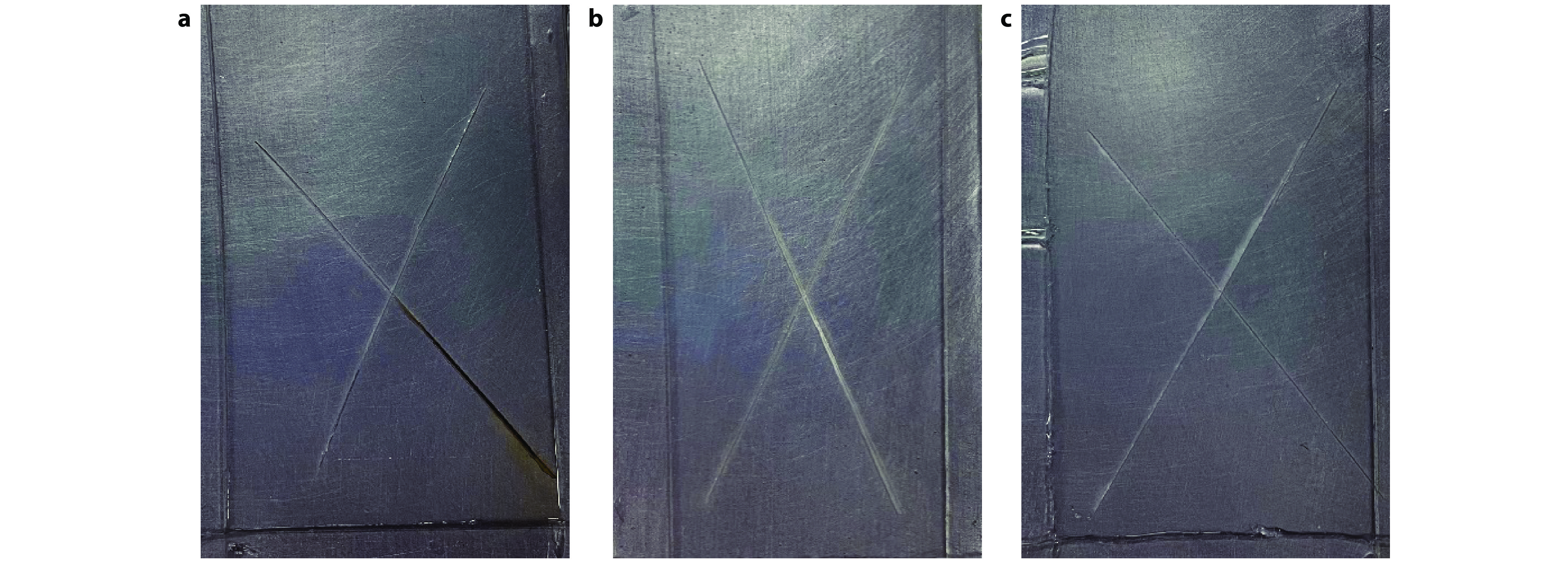
Figure 11.
Scratch immersion test images of a APTES@Kaolin/EP, b SiO2@AK/EP and c 8-HQ@SiO2@AK/EP coatings.
-
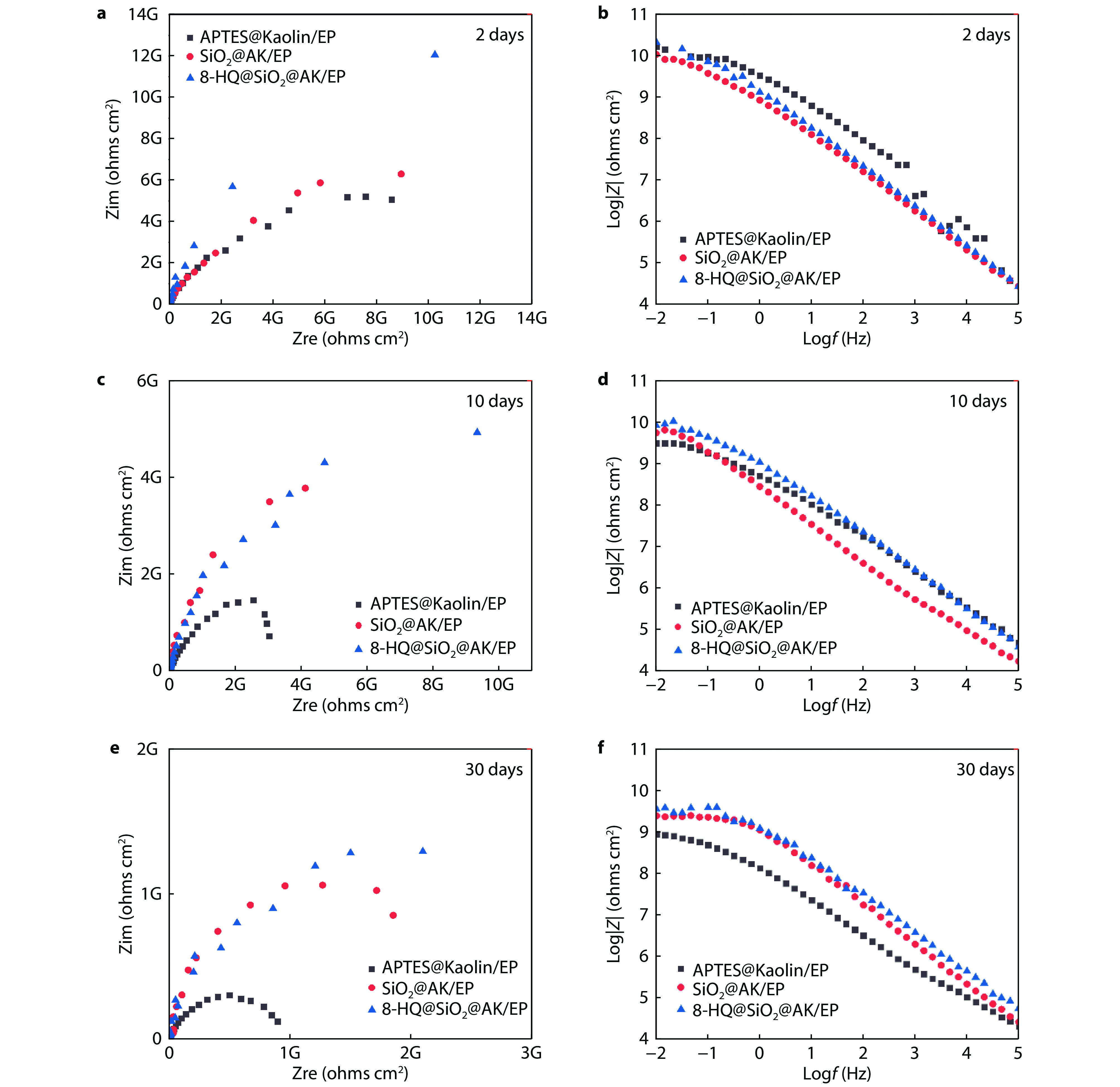
Figure 12.
The Nyquist and Bode diagrams of 8-HQ@SiO2@AK/EP coating immersing in 3.5% NaCl solution for a-b 2 days, c-d 10 days and e-f 30 days.
-
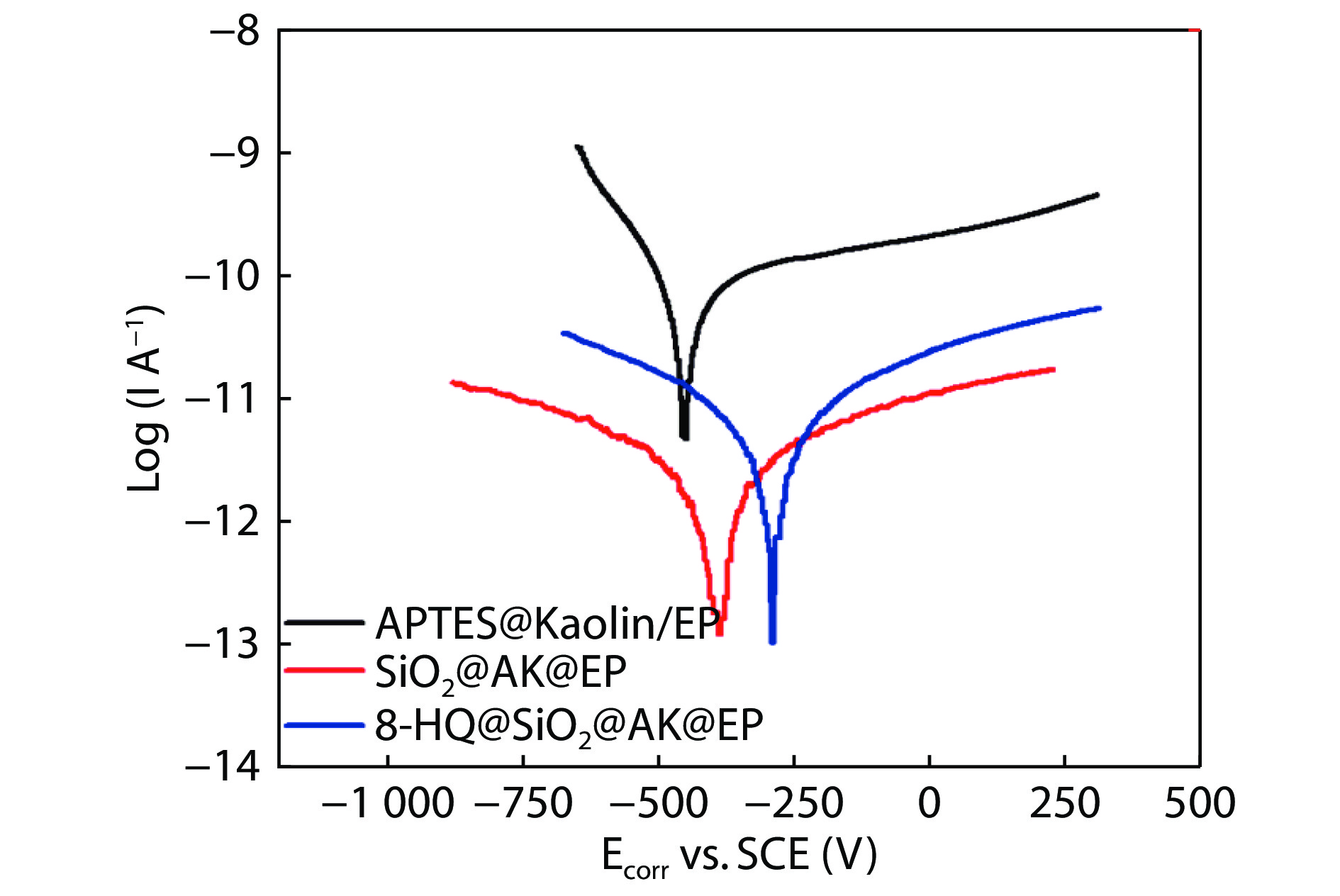
Figure 13.
Polarization curves of APTES@Kaolin/EP, SiO2@AK/EP and 8-HQ@SiO2@AK/EP coatings immersed in 3.5% NaCl solution for 30 days.

 DownLoad:
DownLoad: