| Citation: | Siqi Jiang, Dayong Ren, Yaojie Lei, Hongli Suo, Wei-Hong Lai. Mechanisms of oxygen evolution reaction in metal oxides: adsorbate evolution mechanism versus lattice oxygen mechanism[J]. Materials Lab, 2023, 2(2): 220054. doi: 10.54227/mlab.20220054 |
Mechanisms of oxygen evolution reaction in metal oxides: adsorbate evolution mechanism versus lattice oxygen mechanism
-
Abstract
Water electrolysis provides a promising technology for hydrogen production, but the sluggish four-electron conversion-process of the oxygen evolution reaction results in high overpotential and a low efficiency of water splitting. To rationalize and improve the performance of oxygen evolution reaction, it is crucial to understand the electrochemical mechanisms occurring in cells and monitor the structural changes of newly developed catalysts. As the most recognized mechanisms, the adsorbate evolution mechanism and the lattice oxygen mechanism have been utilized to explain the physical and chemical behaviors of the oxygen evolution reaction. Thus, we herein provide a perspective on these two paths by summarizing the recent progresses in oxygen evolution reactions and building fundamental connections between material designs and the two mechanisms. Insights from this work offer solution to address the current challenges and limitations for the water oxidation.
-
Keywords:
- Oxygen evolution reaction /
- AEM /
- LOM /
- Electrocatalysis
-
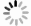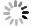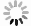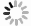
-
References
1. M. Gong, H. Dai, Nano. Res., 2014, 8, 23 2. S. Chu, Y. Cui, N. Liu, Nat. Mater., 2017, 16, 16 3. M. Z. Jacobson, W. Colella, D. Golden, Sci., 2005, 308, 1901 4. X. Yang, Y. Wang, C. M. Li, D. Wang, Nano. Res., 2021, 14, 3446 5. Y. Yang, Y. Yang, Y. Liu, S. Zhao, Z. Tang, Small Science, 2021, 1, 2100015 6. S. Zhao, C. Tan, C.-T. He, P. An, F. Xie, S. Jiang, Y. Zhu, K.-H. Wu, B. Zhang, H. Li, J. Zhang, Y. Chen, S. Liu, J. Dong, Z. Tang, Nat. Energy., 2020, 5, 881 7. L. Tian, X. Zhai, X. Wang, X. Pang, J. Li, Z. Li, Electrochim. Acta, 2020, 337, 135823 8. N. C. S. Selvam, L. Du, B. Y. Xia, P. J. Yoo, B. You, Adv. Funct. Mater., 2021, 31, 2008190 9. S. Xu, H. Zhao, T. Li, J. Liang, S. Lu, G. Chen, S. Gao, A. M. Asiri, Q. Wu, X. Sun, J. Mater. Chem. A, 2020, 8, 19729 10. X. Zheng, P. Li, S. Dou, W. Sun, H. Pan, D. Wang, Y. Li, Energy Environ. Sci., 2021, 14, 2809 11. T. Wang, L. Tao, X. Zhu, C. Chen, W. Chen, S. Du, Y. Zhou, B. Zhou, D. Wang, C. Xie, P. Long, W. Li, Y. Wang, R. Chen, Y. Zou, X.-Z. Fu, Y. Li, X. Duan, S. Wang, Nat. Catal., 2022, 5, 66 12. Q. Zhang, N. M. Bedford, J. Pan, X. Lu, R. Amal, Adv. Energy. Mater., 2019, 9, 1901312 13. J. P. Hughes, J. Clipsham, H. Chavushoglu, S. J. Rowley-Neale, C. E. Banks, Renewable and Sustainable Energy Reviews, 2021, 139, 110709 14. V. I. Birss, A. Damjanovic, P. Hudson, J. Electrochem. Soc., 1986, 133, 1621 15. B. E. Conway, T. Liu, Langmuir, 1990, 6, 268 16. L. Li, P. Wang, Q. Shao, X. Huang, Adv. Mater., 2021, 33, 2004243 17. F. Lyu, Q. Wang, S. M. Choi, Y. Yin, Small, 2019, 15, 1804201 18. S. Sultan, J. N. Tiwari, A. N. Singh, S. Zhumagali, M. Ha, C. W. Myung, P. Thangavel, K. S. Kim, Adv. Energy. Mater., 2019, 9, 1900624 19. F.-Y. Chen, Z.-Y. Wu, Z. Adler, H. Wang, Joule, 2021, 5, 1704 20. Y. Sun, H. Liao, J. Wang, B. Chen, S. Sun, S. J. H. Ong, S. Xi, C. Diao, Y. Du, J.-O. Wang, M. B. H. Breese, S. Li, H. Zhang, Z. J. Xu, Nat. Catal., 2020, 3, 554 21. Y. Jiao, Y. Zheng, M. Jaroniec, S. Z. Qiao, Chem. Soc. Rev., 2015, 44, 2060 22. B. M. Hunter, H. B. Gray, A. M. Muller, Chem. Rev., 2016, 116, 14120 23. J. Song, C. Wei, Z.-F. Huang, C. Liu, L. Zeng, X. Wang, Z. J. Xu, Chem. Soc. Rev., 2020, 49, 2196 24. F. Song, L. Bai, A. Moysiadou, S. Lee, C. Hu, L. Liardet, X. Hu, J. Am. Chem. Soc., 2018, 140, 7748 25. N.-T. Suen, S.-F. Hung, Q. Quan, N. Zhang, Y.-J. Xu, H. M. Chen, Chem. Soc. Rev., 2017, 46, 337 26. X. Rong, J. Parolin, A. M. Kolpak, Acs. Catal., 2016, 6, 1153 27. D. A. Kuznetsov, M. A. Naeem, P. V. Kumar, P. M. Abdala, A. Fedorov, C. R. Müller, J. Am. Chem. Soc., 2020, 142, 7883 28. J. H. Montoya, L. C. Seitz, P. Chakthranont, A. Vojvodic, T. F. Jaramillo, J. K. Nørskov, Nat. Mater., 2017, 16, 70 29. X. Wang, H. Zhong, S. Xi, W. S. V. Lee, J. Xue, Adv. Mater., 2022, 34, 2107956 30. N. Zhang, Y. Chai, Energy Environ. Sci., 2021, 14, 4647 31. Z.-F. Huang, J. Song, Y. Du, S. Xi, S. Dou, J. M. V. Nsanzimana, C. Wang, Z. J. Xu, X. Wang, Nat. Energy., 2019, 4, 329 32. W.-H. Lai, L.-F. Zhang, W.-B. Hua, S. Indris, Z.-C. Yan, Z. Hu, B. Zhang, Y. Liu, L. Wang, M. Liu, R. Liu, Y.-X. Wang, J.-Z. Wang, Z. Hu, H.-K. Liu, S.-L. Chou, S.-X. Dou, Angew. Chem. Int. Ed., 2019, 58, 11868 33. Y.-Q. Zhou, L. Zhang, H.-L. Suo, W. Hua, S. Indris, Y. Lei, W.-H. Lai, Y.-X. Wang, Z. Hu, H.-K. Liu, S.-L. Chou, S.-X. Dou, Adv. Funct. Mater., 2021, 31, 2101797 34. N. Ran, E. Song, Y. Wang, Y. Zhou, J. Liu, Energy Environ. Sci., 2022, 15, 2071 35. N. B. Halck, V. Petrykin, P. Krtil, J. Rossmeisl, PCCP, 2014, 16, 13682 36. H. Fei, J. Dong, Y. Feng, C. S. Allen, C. Wan, B. Volosskiy, M. Li, Z. Zhao, Y. Wang, H. Sun, P. An, W. Chen, Z. Guo, C. Lee, D. Chen, I. Shakir, M. Liu, T. Hu, Y. Li, A. I. Kirkland, X. Duan, Y. Huang, Nat. Catal., 2018, 1, 63 37. A. D. Doyle, J. H. Montoya, A. Vojvodic, ChemCatChem, 2015, 7, 738 38. Z. Kou, X. Li, L. Zhang, W. Zang, X. Gao, J. Wang, Small Science, 2021, 1, 2100011 39. J. Shan, Y. Zheng, B. Shi, K. Davey, S.-Z. Qiao, ACS. Energy. Lett., 2019, 4, 2719 40. E. Fabbri, M. Nachtegaal, T. Binninger, X. Cheng, B.-J. Kim, J. Durst, F. Bozza, T. Graule, R. Schäublin, L. Wiles, M. Pertoso, N. Danilovic, K. E. Ayers, T. J. Schmidt, Nat. Mater., 2017, 16, 925 41. D. Friebel, M. W. Louie, M. Bajdich, K. E. Sanwald, Y. Cai, A. M. Wise, M.-J. Cheng, D. Sokaras, T.-C. Weng, R. Alonso-Mori, R. C. Davis, J. R. Bargar, J. K. Nørskov, A. Nilsson, A. T. Bell, J. Am. Chem. Soc., 2015, 137, 1305 42. Q. Yin, J. M. Tan, C. Besson, Y. V. Geletii, D. G. Musaev, A. E. Kuznetsov, Z. Luo, K. I. Hardcastle, C. L. Hill, Sci., 2010, 328, 342 43. F. M. Toma, A. Sartorel, M. Iurlo, M. Carraro, P. Parisse, C. Maccato, S. Rapino, B. R. Gonzalez, H. Amenitsch, T. Da Ros, L. Casalis, A. Goldoni, M. Marcaccio, G. Scorrano, G. Scoles, F. Paolucci, M. Prato, M. Bonchio, Nat. Chem., 2010, 2, 826 44. B. Zhang, X. Zheng, O. Voznyy, R. Comin, M. Bajdich, M. García-Melchor, L. Han, J. Xu, M. Liu, L. Zheng, F. P. García de Arquer, C. T. Dinh, F. Fan, M. Yuan, E. Yassitepe, N. Chen, T. Regier, P. Liu, Y. Li, P. De Luna, A. Janmohamed, H. L. Xin, H. Yang, A. Vojvodic, E. H. Sargent, Sci., 2016, 352, 333 45. C. Wang, L. Jin, H. Shang, H. Xu, Y. Shiraishi, Y. Du, Chin. Chem. Lett., 2021, 32, 2108 46. A. Zagalskaya, V. Alexandrov, Acs. Catal., 2020, 10, 3650 47. X. Wang, C. Xing, Z. Liang, P. Guardia, X. Han, Y. Zuo, J. Llorca, J. Arbiol, J. Li, A. Cabot, J. Mater. Chem. A., 2022, 10, 3659 48. L. An, C. Wei, M. Lu, H. Liu, Y. Chen, G. G. Scherer, A. C. Fisher, P. Xi, Z. J. Xu, C.-H. Yan, Adv. Mater., 2021, 33, 2006328 49. L. Zhang, H. Jang, H. Liu, M. G. Kim, D. Yang, S. Liu, X. Liu, J. Cho, Angew. Chem. Int. Ed., 2021, 60, 18821 50. K. Zhu, F. Shi, X. Zhu, W. Yang, Nano Energy, 2020, 73, 104761 51. J. Hwang, Z. Feng, N. Charles, X. R. Wang, D. Lee, K. A. Stoerzinger, S. Muy, R. R. Rao, D. Lee, R. Jacobs, D. Morgan, Y. Shao-Horn, Mater. Today, 2019, 31, 100 52. A. Grimaud, O. Diaz-Morales, B. Han, W. T. Hong, Y.-L. Lee, L. Giordano, K. A. Stoerzinger, M. T. M. Koper, Y. Shao-Horn, Nat. Chem., 2017, 9, 457 53. P. Wang, Q. Cheng, C. Mao, W. Su, L. Yang, G. Wang, L. Zou, Y. Shi, C. Yan, Z. Zou, H. Yang, J. Power Sources, 2021, 502, 229903 54. W. T. Hong, K. A. Stoerzinger, Y.-L. Lee, L. Giordano, A. Grimaud, A. M. Johnson, J. Hwang, E. J. Crumlin, W. Yang, Y. Shao-Horn, Energy Environ. Sci., 2017, 10, 2190 55. Z.-F. Huang, S. Xi, J. Song, S. Dou, X. Li, Y. Du, C. Diao, Z. J. Xu, X. Wang, Nat. Commun., 2021, 12, 3992 56. H. Liu, X. Li, C. Peng, L. Zhu, Y. Zhang, H. Cheng, J. Cui, Q. Wu, Y. Zhang, Z. Chen, W. Zou, W. Gu, H. Huang, J. Wang, B. Ye, Z. Fu, Y. Lu, J. Mater. Chem. A., 2020, 8, 13150 57. T. Binninger, R. Mohamed, K. Waltar, E. Fabbri, P. Levecque, R. Kötz, T. J. Schmidt, Scientific Reports, 2015, 5, 12167 58. P. Thangavel, M. Ha, S. Kumaraguru, A. Meena, A. N. Singh, A. M. Harzandi, K. S. Kim, Energy Environ. Sci., 2020, 13, 3447 59. W. E. Mustain, P. A. Kohl, Nat. Energy., 2020, 5, 359 60. D. Li, E. J. Park, W. Zhu, Q. Shi, Y. Zhou, H. Tian, Y. Lin, A. Serov, B. Zulevi, E. D. Baca, C. Fujimoto, H. T. Chung, Y. S. Kim, Nat. Energy., 2020, 5, 378 61. J. Gao, C.-Q. Xu, S.-F. Hung, W. Liu, W. Cai, Z. Zeng, C. Jia, H. M. Chen, H. Xiao, J. Li, Y. Huang, B. Liu, J. Am. Chem. Soc., 2019, 141, 3014 62. K. Zhu, X. Zhu, W. Yang, Angew. Chem. Int. Ed., 2019, 58, 1252 -
Rights and permissions
This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.
Information
Article Metrics
-
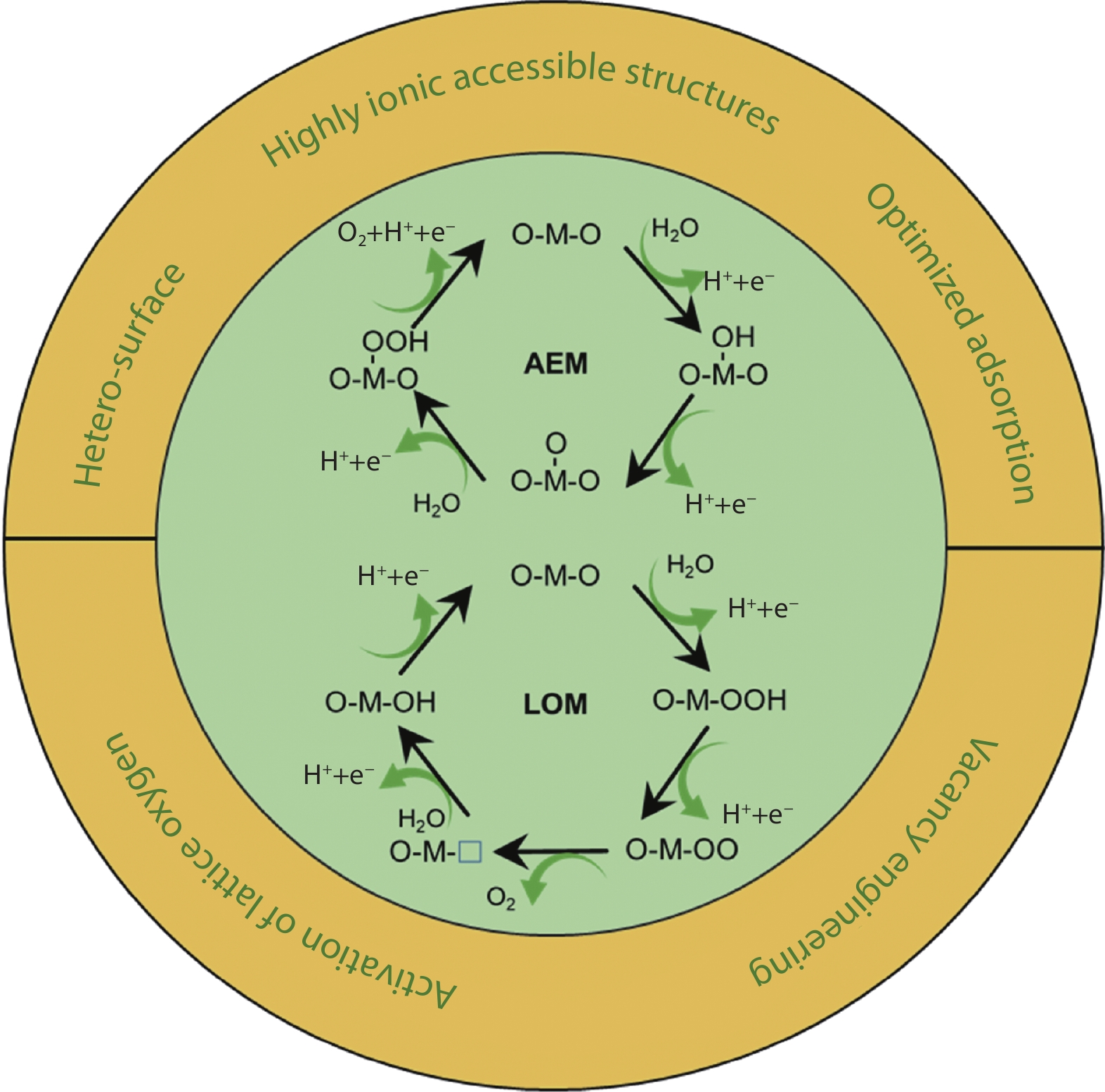
Figure 1.
a Schematic diagram of the AEM. b Schematic diagram of the LOM.[19] Copyright 2021, Elsevier. c The scaling relationships between the reaction mechanism diagrams of AEM and LOM with energy bands. The formation of a density of states (DOS) diagram for spinel oxide with the contributions from tetragonal cations (MT), octahedral cations (MO) and oxygen anions (O).[20] Copyright 2020, Springer Nature.
-
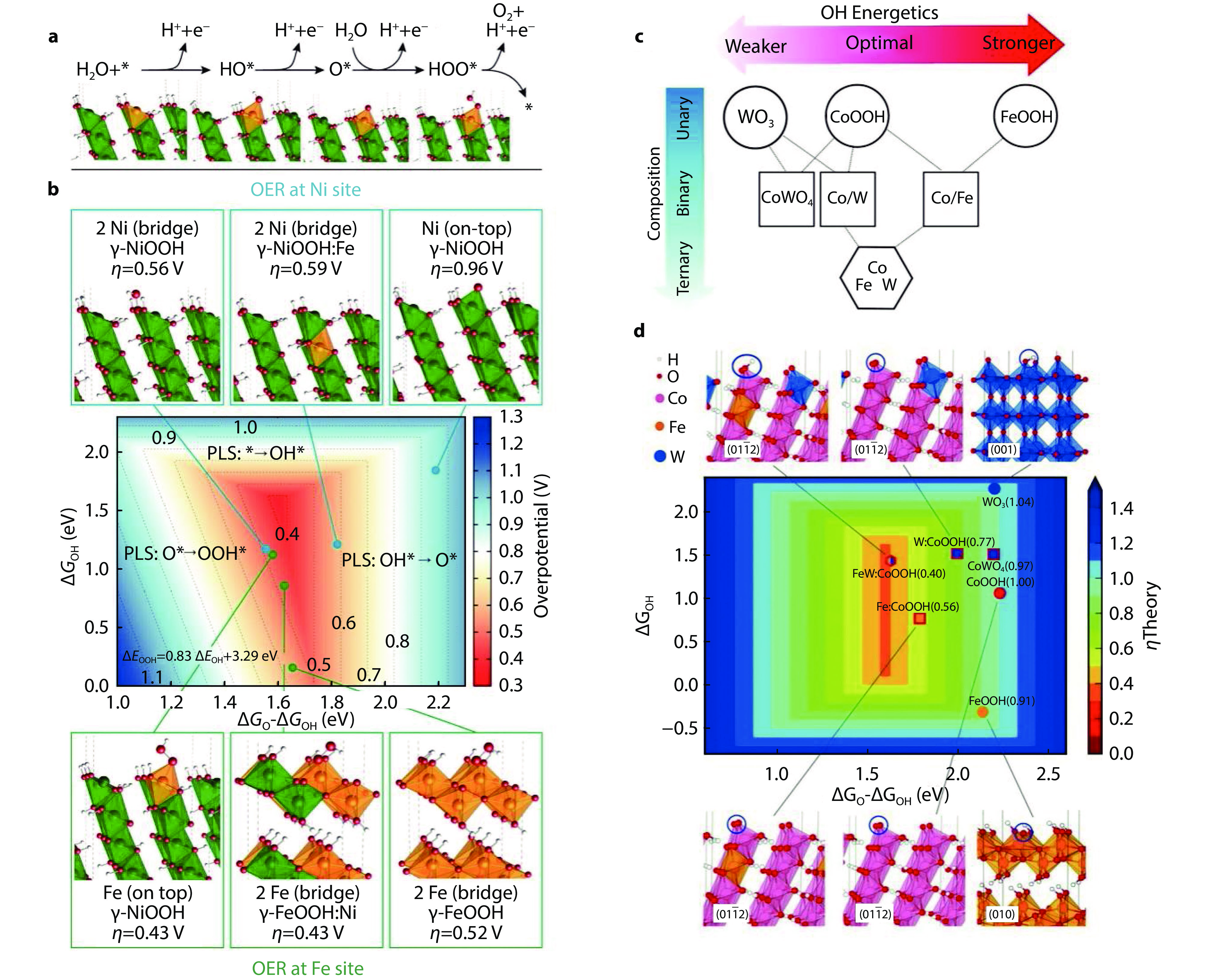
Figure 2.
a OER pathway for intermediates. b The overpotential of the Gibbs free energy function of OER intermediates.[41] Copyright 2015, American Chemical Society. c Variation of OH adsorption energy of WO3, CoOOH, FeOOH and CoWO4. d Density functional theory calculations for OER activity of pure Fe, Co hydroxyl oxides and W, Fe-doped Co hydroxyl oxides.[44] Copyright 2016, American Association for the Advancement of Science.
-
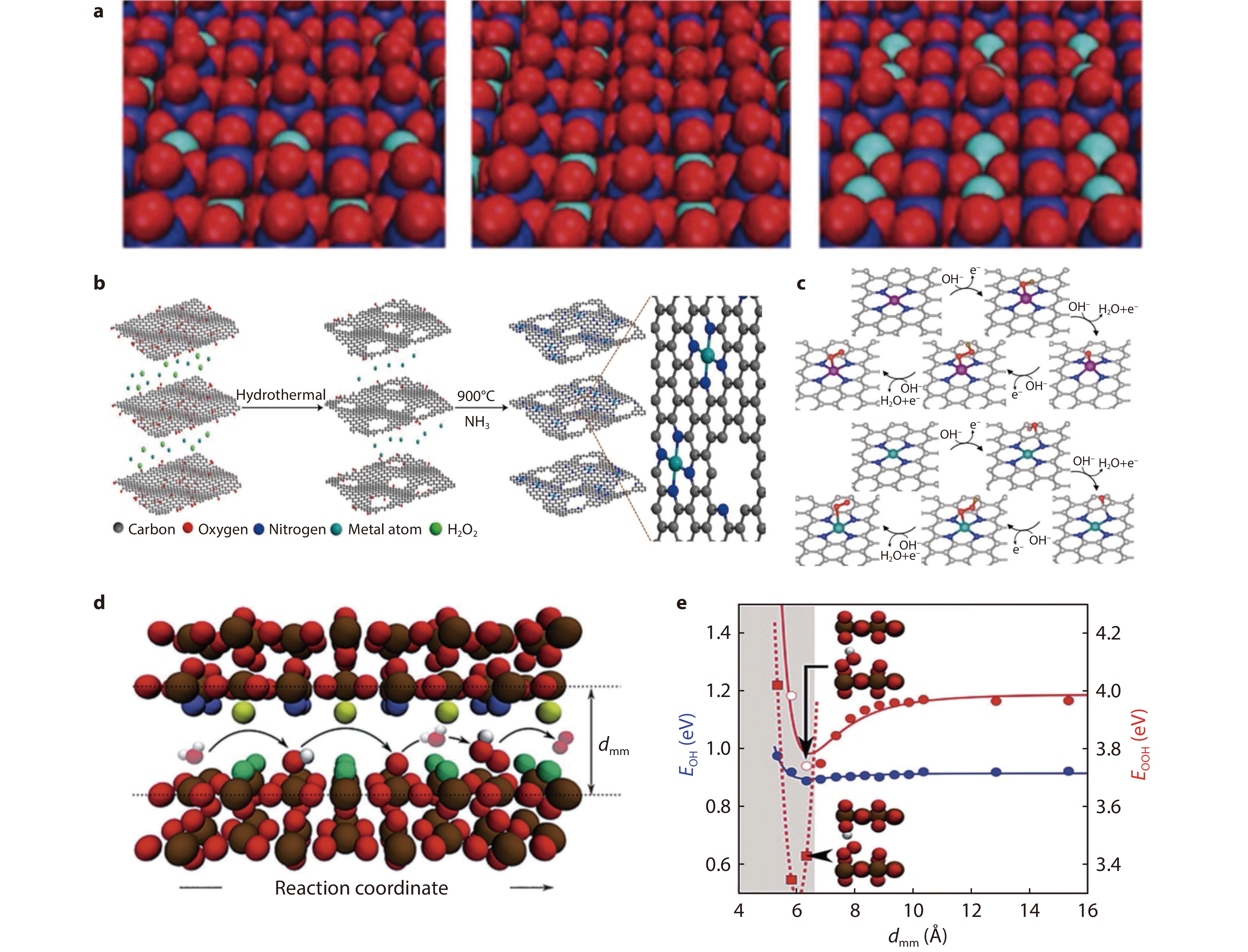
Figure 3.
a Schematic diagram of the three possible active sites for Ni-doped Ru.[35] Copyright 2014, Royal Society of Chemistry. b Schematic diagram of M-NHGFs. c Optimal geometry of the single-site and double-site mechanisms of OER.[36] Copyright 2018, Macmillan Publishers Limited. d Atomistic side view of the model system used to simulate confinement. e Adsorption energy of OER intermediates as a function of surface channel width.[37] Copyright 2014, Wiley-VCH.
-
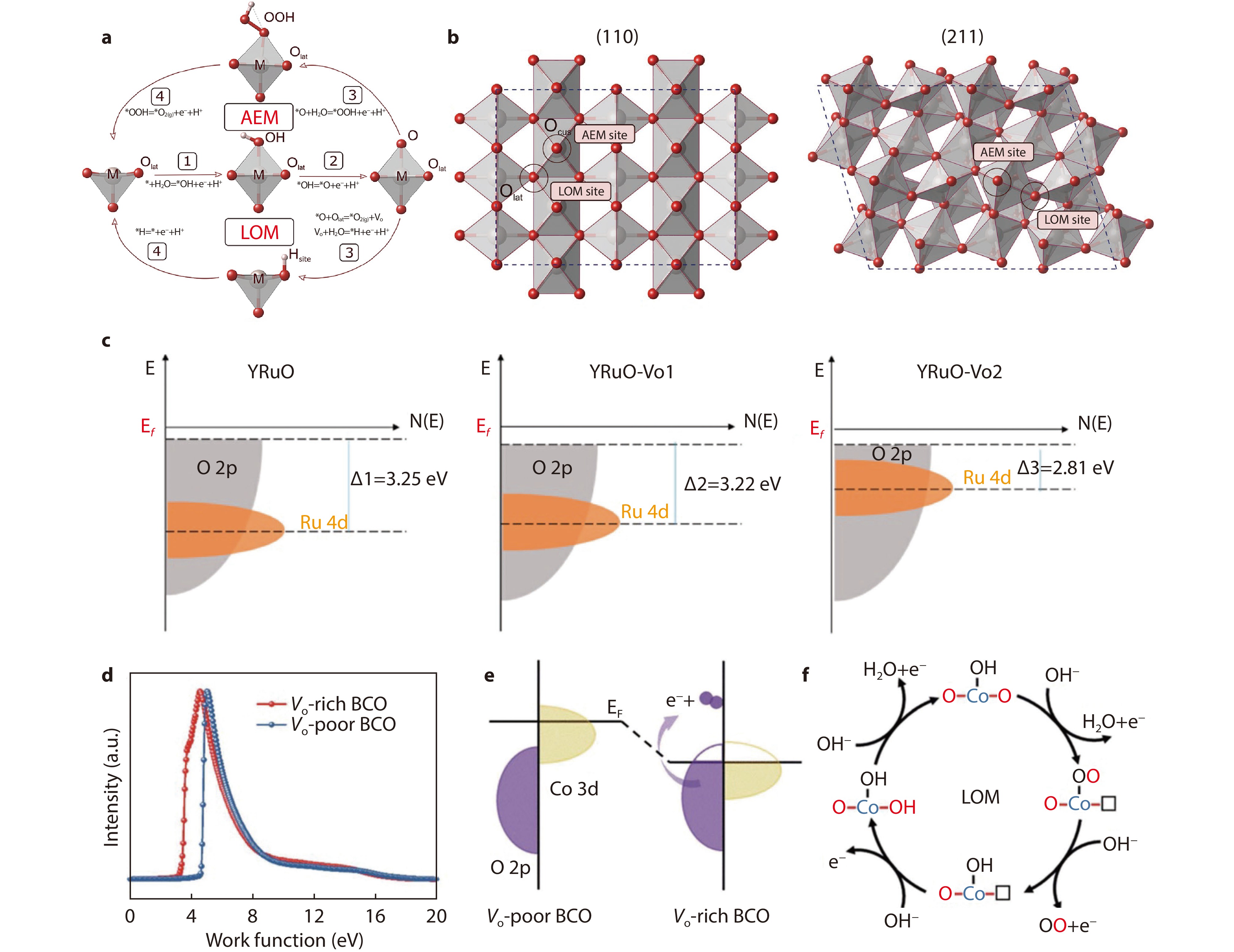
Figure 4.
a Schematic representation of the AEM and LOM. b Top view of (110) and (211) surfaces of rutile-structured RuO2 and IrO2 employed in DFT calculations of the OER overpotentials.[46] Copyright 2020, American Chemical Society. c Schematic rigid band diagrams of Y2Ru2O7-δ-xFx.[53] Copyright 2021, Elsevier. d Ultraviolet-photoelectron spectra of oxygen-vacancy-rich (Vo-rich) and oxygen-vacancy-poor (Vo-poor) BCO. e Schematic diagram of the energy bands of Vo-rich and Vo-poor BCO. f Schematic diagram of the LOM of BCO.[56] Copyright 2020, The Royal Society of Chemistry.
-
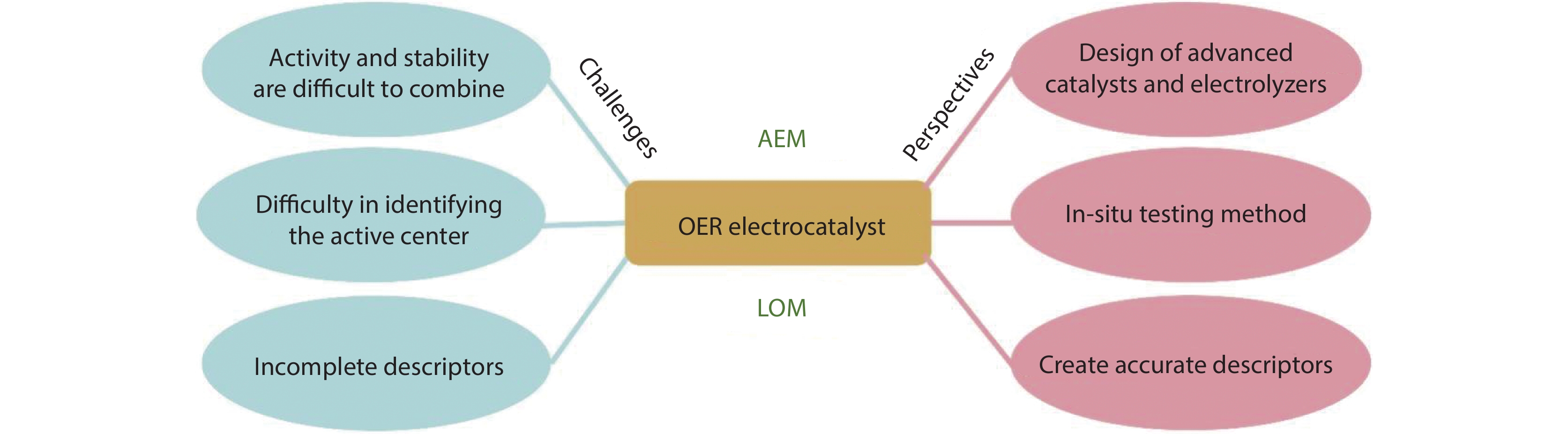
Figure 5.
Challenges and opportunities for OER electrocatalysts following the AEM and LOM mechanisms.

 Siqi Jiang is a master student in the Faculty of Materials and Manufacturing, Beijing University of Technology. Her research focuses on designs of lithium metal oxides and transition metal-based hydroxides for efficient oxygen evolution reactions.
Siqi Jiang is a master student in the Faculty of Materials and Manufacturing, Beijing University of Technology. Her research focuses on designs of lithium metal oxides and transition metal-based hydroxides for efficient oxygen evolution reactions.  Hongli Suo is a distinguished professor and the group leader of High-temperature Superconductor Lab in Beijing University of Technology. She worked as a Postdoctoral Researcher in the Department of Condensed Matter Physics and Applied Physics at the University of Geneva, Switzerland. Suo demonstrates a strong multidisciplinary research background in the fields of materials science, metallurgy, chemistry, and condensed matter physics. Her thesis was selected as the top 100 National Excellent Doctoral Dissertation of P.R. China. She has also published more than 200 scientific papers and nearly 140 of them are listed by mainstream ISI Web of Science.
Hongli Suo is a distinguished professor and the group leader of High-temperature Superconductor Lab in Beijing University of Technology. She worked as a Postdoctoral Researcher in the Department of Condensed Matter Physics and Applied Physics at the University of Geneva, Switzerland. Suo demonstrates a strong multidisciplinary research background in the fields of materials science, metallurgy, chemistry, and condensed matter physics. Her thesis was selected as the top 100 National Excellent Doctoral Dissertation of P.R. China. She has also published more than 200 scientific papers and nearly 140 of them are listed by mainstream ISI Web of Science.  Wei-Hong Lai
Wei-Hong Lai 
 DownLoad:
DownLoad: