| Citation: | Anqi Hu, Yingzheng Hong, Shilun Feng, Shengtai Bian. Wearable Sweat Biosensors on Sports Analysis. Materials Lab 2022, 1, 220028. doi: 10.54227/mlab.20220028 |
Wearable Sweat Biosensors on Sports Analysis
-
Abstract
Wearable sensors provide methods of real-time and non-invasive monitoring of physiological status or motion for sports analytics. Still, these devices relatively have room for improvement, especially in the underexplored field of advanced material and sensing strategy. Here, we present a systematic review of wearable biosensing technology in sports analysis with a focus on materials and sensing modalities with a summary of unresolved challenges and opportunities researchers will be interested in for the future. With a deep understanding of wearable biosensing technologies, advanced wearable biosensors would have a significant impact on athletic monitoring and sports analysis.
-
Keywords:
- Wearable biosensor /
- Microfluidics /
- Sports analytics
-
-
References
1. L. Wang, T. Xu, C. Fan, X. Zhang, iScience, 2020, 24, 102028 2. Z. Cao, R. Wang, T. He, F. Xu, J. Sun, ACS Appl. Mater. Interfaces, 2018, 10, 14087 3. R. D. Munje, S. Muthukumar, S. Prasad, Sens. Actuators B Chem., 2017, 238, 482 4. L. Nayak, S. Mohanty, S. K. Nayak, A. Ramadoss, J. Mater. Chem. C, 2019, 7, 8771 5. J. Cai, Q. Wang, X. Li, Y. Guan, L. He, S. Yan, R. You, Q. Zhang, Mater. Today Commun., 2020, 22, 100776 6. B. Joonwon, P. Jongnam, K. Seongsoo, C. Hana, J. K. Hye, P. Soyeon, S. S. Dong, J. Ind. Eng. Chem., 2020, 89, 1 7. Y. Y. Choi, D. H. Ho, J. H. Cho, ACS Appl. Mater. Interfaces, 2020, 12, 9824 8. C. H. Li, C. Wang, C. Keplinger, J. L. Zuo, L. Jin, Y. Sun, P. Zheng, Y. Cao, F. Lissel, C. Linder, X. Z. You, Z. Bao, Nat. Chem., 2016, 8, 618 9. R. Yu, T. Xia, B. Wu, J. Yuan, L. Ma, G. J. Cheng, F. Liu, ACS Appl. Mater. Interfaces, 2020, 12, 35291 10. P. Zhang, Y. Chen, Y. Li, Y. Zhang, J. Zhang, L. Huang, Sensors, 2020, 20, 1154 11. B. Yin, Y. Wen, T. Hong, Z. Xie, G. Yuan, Q. Ji, H. Jia, ACS Appl. Mater. Interfaces, 2017, 9, 32054 12. Y. Zheng, R. Yin, Y. Zhao, H. Liu, C. Shen, Chem. Eng. J., 2021, 420, 127720 13. M. Amjadi, K.-U. Kyung, I. Park, M. Sitti, Adv. Funct. Mater., 2016, 26, 1678 14. J. Chen, J. Zhang, Z. Luo, J. Zhang, L. Li, Y. Su, X. Gao, Y. Li, W. Tang, C. Cao, Q. Liu, L. Wang, H. Li, ACS Appl. Mater. Interfaces, 2020, 12, 22200 15. S. Ye, S. Feng, L. Huang, S. Bian, Biosensors, 2020, 10, 205 16. S. Choi, S. I. Han, D. Kim, T. Hyeon, D. H. Kim, Chem. Soc. Rev., 2019, 48, 1566 17. X. Zhang, W. Lu, G. Zhou, Q. Li, Adv. Mater., 2020, 32, 1902028 18. G. Li, D. W. Lee, Lab Chip, 2017, 17, 3415 19. T. Shay, O. D. Velev, M. D. Dickey, Soft Matter, 2018, 14, 3296 20. G. Yun, S. Y. Tang, S. Sun, D. Yuan, Q. Zhao, L. Deng, S. Yan, H. Du, M. D. Dickey, W. Li, Nat. Commun., 2019, 10, 1300 21. E. J. Markvicka, M. D. Bartlett, X. Huang, C. Majidi, Nat. Mater., 2018, 17, 618 22. M. Gharakhloo, D. Jagleniec, J. Romanski, M. Karbarz, J. Mater. Chem. B., 2022, 10, 4463 23. L. Lu, B. Yang, Y. Zhai, J. Liu, Nano Energy, 2020, 76, 104966 24. A. Chortos, J. Liu, Z. Bao, Nat. Mater., 2016, 15, 937 25. X. Jing, H. Mi, Y. Lin, E. Enriquez, X. Peng, L. Turng, ACS Appl. Mater. Interfaces., 2018, 10, 20897 26. J. Yeo, Kenry, J. Yu, K. Loh, Z. Wang, C. Lim, ACS Sensors, 2016, 1, 543 27. Y. Zhang, H. Guo, S. B. Kim, Y. Wu, D. Ostojich, S. H. Park, X. Wang, Z. Weng, R. Li, A. J. Bandodkar, Y. Sekine, J. Choi, S. Xu, S. Quaggin, R. Ghaffari, J. A. Rogers, Lab Chip, 2019, 19, 1545 28. J. Choi, A. J. Bandodkar, J. T. Reeder, T. R. Ray, A. Turnquist, S. B. Kim, N. Nyberg, A. Hourlier-Fargette, J. B. Model, A. J. Aranyosi, S. Xu, R. Ghaffari, J. A. Rogers, ACS Sensors, 2019, 4, 379 29. R. Olivia, W. Leon, F. Mike, ACM Designing Interactive Systems Conference, Electr Network, June 2021. 30. P. Ivan, W. G. Nan F. Shiho, E. K. Mustafa, S. Carsten, E. R. Karen, 34th Annual CHI Conference on Human Factors in Computing Systems, San Jose, CA, May 2016. 31. V. Mazzaracchio, L. Fiore, S. Nappi, G. Marrocco, F. Arduini, Talanta, 2021, 222, 121502 32. Y. Song, J. Min, Y. Yu, H. Wang, Y. Yang, H. Zhang, W. Gao, Sci. Adv., 2020, 6, eaay9842 33. J. Choi, Y. Xue, W. Xia, T. R. Ray, J. T. Reeder, A. J. Bandodkar, D. Kang, S. Xu, Y. Huang, J. A. Rogers, Lab Chip, 2017, 17, 2572 34. Q. Duan, Y. Lu, ACS Appl. Mater. Interfaces, 2021, 13, 28832 35. Y. S. Can, N. Chalabianloo, D. Ekiz, C. Ersoy, Sensors, 2019, 19, 1849 36. J. Chen, J. Zhang, Z. Luo, Z. Zhang, L. Li, Y. Su, X. Gao, Y. Li, W. Tang, C. Cao, Q. Liu, L. Wang, H. Li, ACS Appl. Mater. Interfaces, 2020, 19, 22200 37. L. Li, H. Yang, X. Li, S. Yan, A. Xu, R. You, Q. Zhang, Carbohydrate Polymers, 2021, 1, 117214 38. B. Shi, L. Li, A. Chen, T. Jen, X. Liu, G. Shen, Nano-Micro Lett., 2022, 14, 34 39. H. Huang, X. Ning, M. Zhou, T. Sun, X. Wu, X. Zhang, ACS Appl. Mater. Interfaces, 2021, 13, 18021 40. X. Zhao, X. Wen, P. Sun, C. Zeng, M. Liu, F. Yang, H. Bi, D. Li, R. Ma, J. Wang, X. Yu, D. Zhang, H. Lu, ACS Appl. Mater. Interfaces, 2021, 13, 10428 41. A. J. Bandodkar, P. Gutruf, J. Choi, K. Lee, Y. Sekine, J. T. Reeder, W. J. Jeang, A. J. Aranyosi, S. P. Lee, J. B. Model, R. Ghaffari, C. Su, J. P. Leshock, T. Ray, A. Verrillo, K. Thomas, V. Krishnamurthi, S. Han, J. Kim, S. Krishnan, T. Hang, J. A. Rogers, Sci. Adv., 2019, 5, eaav3294 42. S. Seyedin, S. Uzun, A. Levitt, B. Anasori, G. Dion, Y. Gogotsi, J. M. Razal, Adv. Funct. Mater., 2020, 30, 1910504 43. X. Wang, R. Guo, J. Liu, Adv. Mater. Technol., 2018, 4, 1800549 44. Y. Huang, F. Yang, S. Liu, R. Wang, J. Guo, X. Ma, Research, 2021, 2021, 9847285 45. L. Yu, J. C. Yeo, R. H. Soon, T. Yeo, H. H. Lee, C. T. Lim, ACS Appl. Mater. Interfaces, 2018, 10, 12773 -
Rights and permissions
This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.
Information
Article Metrics
-
Figure 1.
(a) Young’s moduli of various substrate materials. The modulus can vary depending on the type of crosslinker, the fraction of polymer component, and curing temperature. The moduli of nanocomposites increase when nanomaterials are embedded in pure elastomer substrates[16]. Copyright 2019, Royal Society of Chemistry. (b,c) Scanning electron microscopy images of the top surface of the chitosan-based films with different Silk nanofibrils/Chitosan ratios. Scale bars: 5 μm. (b-e) cross-section images of the films. Scale bars: 1 μm. (b,d) Pure chitosan, (c,e) Silk nanofibrils/Chitosan ratios = 1/2[37]. Copyright 2021, Elsevier. (f) After electroless nickel plating, the whole acetate fabric was tightddly covered by a nickel layer[34]. Copyright 2019, American Chemical Society.
-
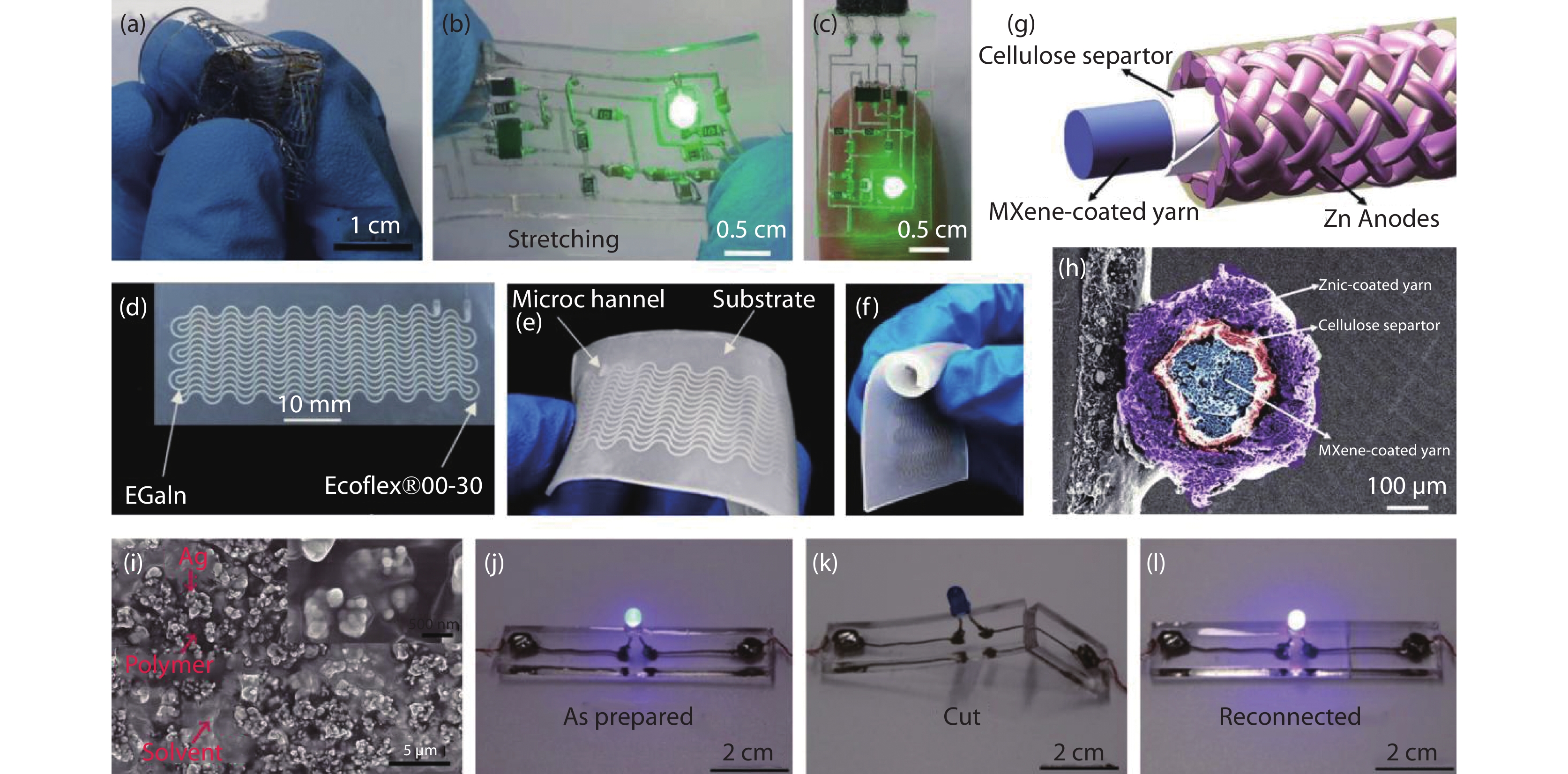
Figure 2.
(a-c) Liquid metal was plated on a silicon dioxide surface and embedded in PDMS as a conductive trace for flexible substrate and a liquid-metal-based stretchable pulse sensor, (a) crumpling deformation, (b) stretching conditions, (c) attached to finger[18]. Copyright 2013, Li. (d-f) An enhanced liquid-metal-based microfluidic strain sensor, (f) bendability of the enhanced strain sensor[36]. Copyright, 2020, American Chemical Society. (g,h) Schematic illustration and Cross-sectional scanning electron microscopy image of MXene and zinc-coated fibers and braided coaxial hybrid fiber supercapacitors. Ti3C2Tx MXene cathode as core electrodes and zinc fiber anode shell was braided on the surface of the Ti3C2Tx fibers across the solid electrolytes[38]. Copyright 2022, Springer Nature. (i) Scanning electron microscopy image of a partially dried conductive ink showing the high-magnification microstructure of Ag aggregates. Ag aggregates surrounded by a layer of polymers (CMC and PAA) are embedded sporadically in the glycerol solvent. (j-l) The LED circuit with self-healing ability[39]. Copyright 2021, American Chemical Society.
-
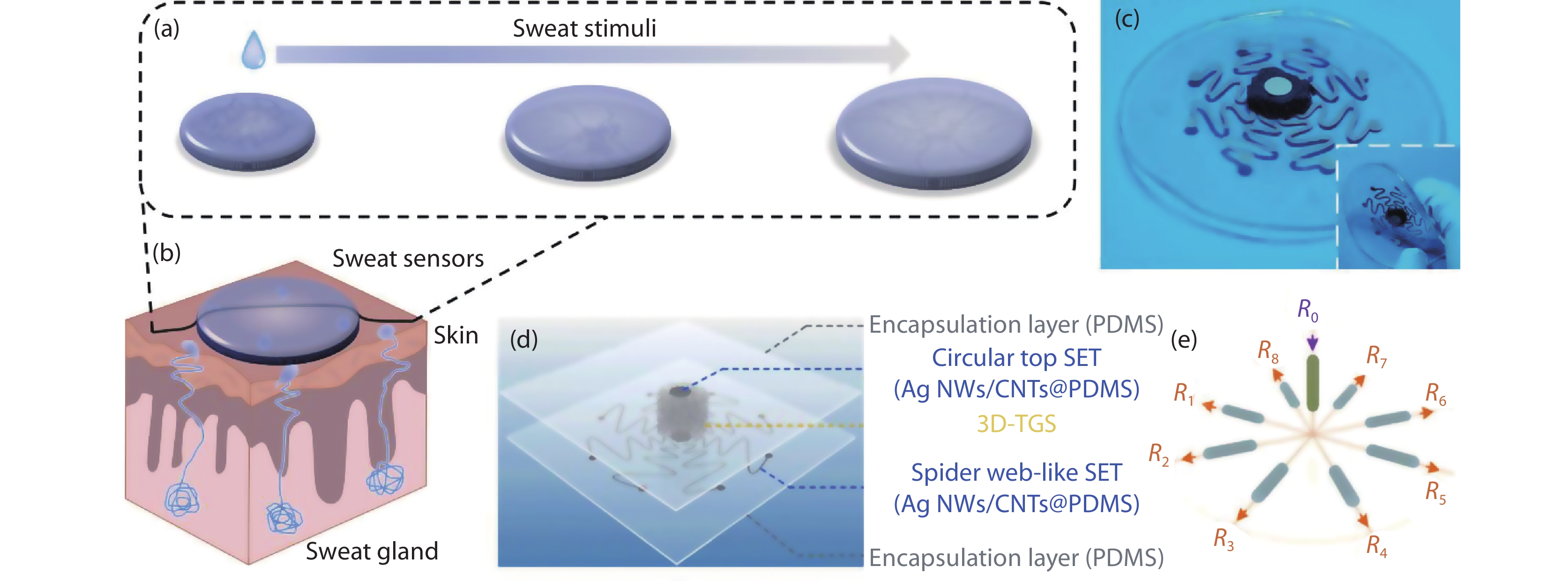
Figure 3.
(a) Schematic diagrams of a superabsorbent hydrogel of a wearable strain sensor for real-time sweat volume monitoring, which shows the swelling process of dry hydrogel to the final equilibrium from left to right. (b) Cross-section view of the structure of sweat glands, the wearable strain sensor is placed across the skin’s surface to absorb the sweat[1]. Copyright 2020, Royal Society of Chemistry. (c) Picture of the spiderweb-like tactile sensor and the inset shows good flexibility. (d) Overall structure image of the sensing device. (e) Image of equivalent circuit of the sensor[40]. Copyright 2021, American Chemical Society.
-
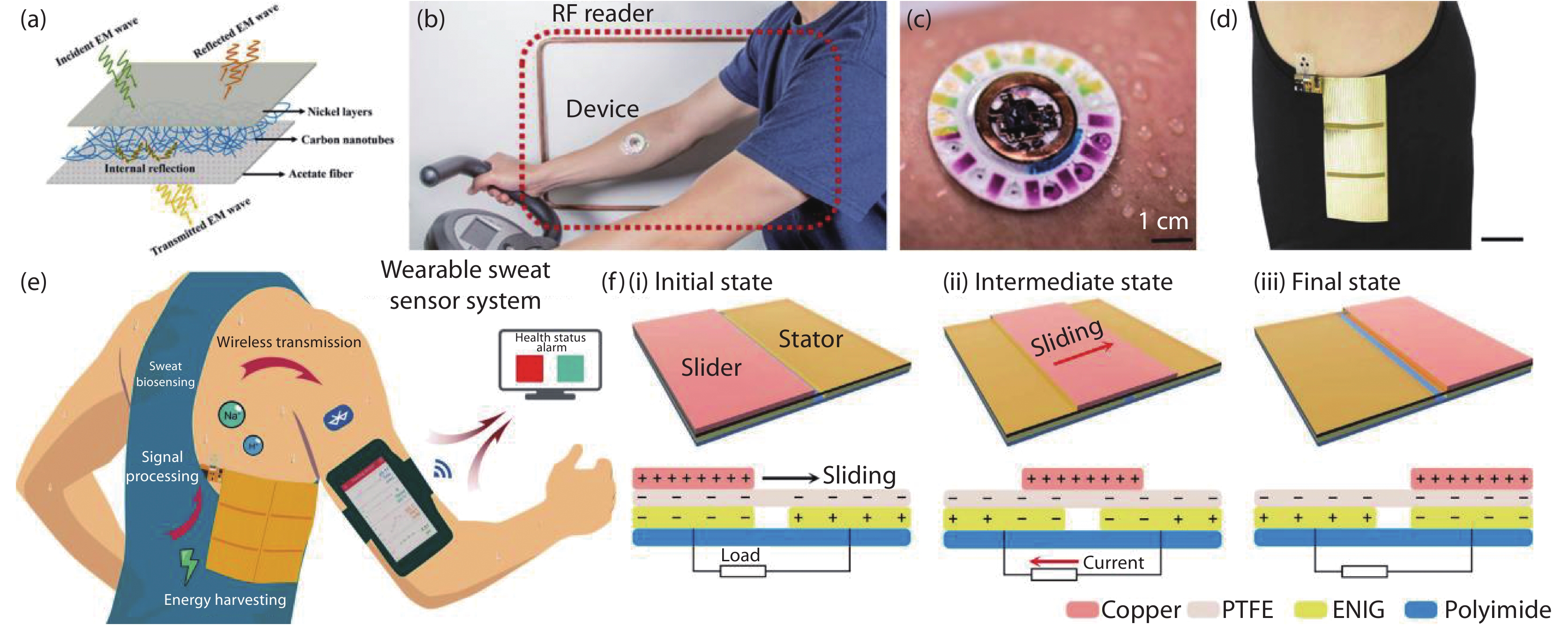
Figure 4.
(a) Schematic description of EMI shielding mechanism for SS@CNTs[34]. Copyright 2019, American Chemical Society. (b) Photograph of a wearable wireless battery-free hybrid sensor system. (c) Image illustrating the device during sweating[41]. Copyright 2019, American Association for the Advancement of Science. (d) Optical images of a flexible printed circuit board (FPCB)-based sensor. Scale bars: 4 cm. (e) Schematic illustrating the sensor that integrates human motion energy harvesting, microfluidic-based sweat biosensing, signal processing, and Bluetooth-based wireless data transmission to a mobile device interface for real-time health status tracking. (f) Schematic illustration of the charge distribution and working mechanism of the freestanding triboelectric nanogenerator (FTENG)[32]. Copyright 2020, American Association for the Advancement of Science.

 Anqi Hu graduated from Beijing Sports University with a bachelor's degree in 2022, he's a researcher of Microfluidics Research & Innovation Laboratory. His recent research focuses on electrochemical sensors for monitoring athletic performance and health condition and wearable microfluidic sensors for sensing of biofluids (e.g., sweat, ISF, and saliva). His current research interests include developing wearable sweat microfluidic sensor, electrochemical sensors for sensing of biofluids and wearable technology.
Anqi Hu graduated from Beijing Sports University with a bachelor's degree in 2022, he's a researcher of Microfluidics Research & Innovation Laboratory. His recent research focuses on electrochemical sensors for monitoring athletic performance and health condition and wearable microfluidic sensors for sensing of biofluids (e.g., sweat, ISF, and saliva). His current research interests include developing wearable sweat microfluidic sensor, electrochemical sensors for sensing of biofluids and wearable technology.  Yingzheng Hong graduated from Xidian University with a bachelor's degree in 2000 and earned a master's degree at University of Science and Technology of China in 2006. Currently, as a Ph.D. candidate at Southeast University, he is also an associate researcher at the Shanghai Fire Research Institute of the Ministry of Emergency Management. Meanwhile, and he is titled as the director of the Key Laboratory of Training and Occupational Health of the Ministry of Emergency Management, a member of the Expert Committee of "China Emergency Rescue" magazine, a distinguished professor of the University of Electronic Science and Technology of China, and a distinguished expert of the University of Electronic Science and Technology of China. His research field locates in digital firefighting equipment, wearable devices and firefighters' occupational health. In 2018, he won the Second Prize of the National Science and Technology Progress Award in this field.
Yingzheng Hong graduated from Xidian University with a bachelor's degree in 2000 and earned a master's degree at University of Science and Technology of China in 2006. Currently, as a Ph.D. candidate at Southeast University, he is also an associate researcher at the Shanghai Fire Research Institute of the Ministry of Emergency Management. Meanwhile, and he is titled as the director of the Key Laboratory of Training and Occupational Health of the Ministry of Emergency Management, a member of the Expert Committee of "China Emergency Rescue" magazine, a distinguished professor of the University of Electronic Science and Technology of China, and a distinguished expert of the University of Electronic Science and Technology of China. His research field locates in digital firefighting equipment, wearable devices and firefighters' occupational health. In 2018, he won the Second Prize of the National Science and Technology Progress Award in this field.  Shilun Feng finished his research fellow journey for POCT microfluidics projects on environmental water in the School of Electrical and Electronic Engineering, Nanyang Technological University, Singapore, in 2020. He completed his Ph.D. in Biomedical POCT microfluidics with Dr. David Inglis, who specialised in POCT microfluidic sampling probe and POCT on-chip cell concentrators, in the School of Engineering, Macquarie University, Australia. He is currently an associate professor in State Key Laboratory of Transducer Technology, Shanghai Institute of Microsystem and Information Technology, Chinese Academy of Sciences, Shanghai. He is focusing on different Point-of-care testing (POCT) researches for food, environmental water, and biomedical sensing. His research interests include biomedical microfluidics; microfabrication; Point-of-care (POC) sampling, manipulation, and testing with the developments of biodevice and instrumentation systems.
Shilun Feng finished his research fellow journey for POCT microfluidics projects on environmental water in the School of Electrical and Electronic Engineering, Nanyang Technological University, Singapore, in 2020. He completed his Ph.D. in Biomedical POCT microfluidics with Dr. David Inglis, who specialised in POCT microfluidic sampling probe and POCT on-chip cell concentrators, in the School of Engineering, Macquarie University, Australia. He is currently an associate professor in State Key Laboratory of Transducer Technology, Shanghai Institute of Microsystem and Information Technology, Chinese Academy of Sciences, Shanghai. He is focusing on different Point-of-care testing (POCT) researches for food, environmental water, and biomedical sensing. His research interests include biomedical microfluidics; microfabrication; Point-of-care (POC) sampling, manipulation, and testing with the developments of biodevice and instrumentation systems.  Shengtai Bian received his Doctor of Engineering in Biomedical Engineering at Tsinghua University. He's currently an associate professor in the School of Sport Science at Beijing Sport University. He's also the principal investigator of Microfluidics Research & Innovation Laboratory. He is focusing on wearable microfluidic sensors for sensing of biofluids (e.g., sweat, ISF, and saliva) and electrochemical sensors for monitoring health condition. His current research interests include high polymer material, wearable technology and Point-of-care testing (POCT) researches for biomedical sensing.
Shengtai Bian received his Doctor of Engineering in Biomedical Engineering at Tsinghua University. He's currently an associate professor in the School of Sport Science at Beijing Sport University. He's also the principal investigator of Microfluidics Research & Innovation Laboratory. He is focusing on wearable microfluidic sensors for sensing of biofluids (e.g., sweat, ISF, and saliva) and electrochemical sensors for monitoring health condition. His current research interests include high polymer material, wearable technology and Point-of-care testing (POCT) researches for biomedical sensing. 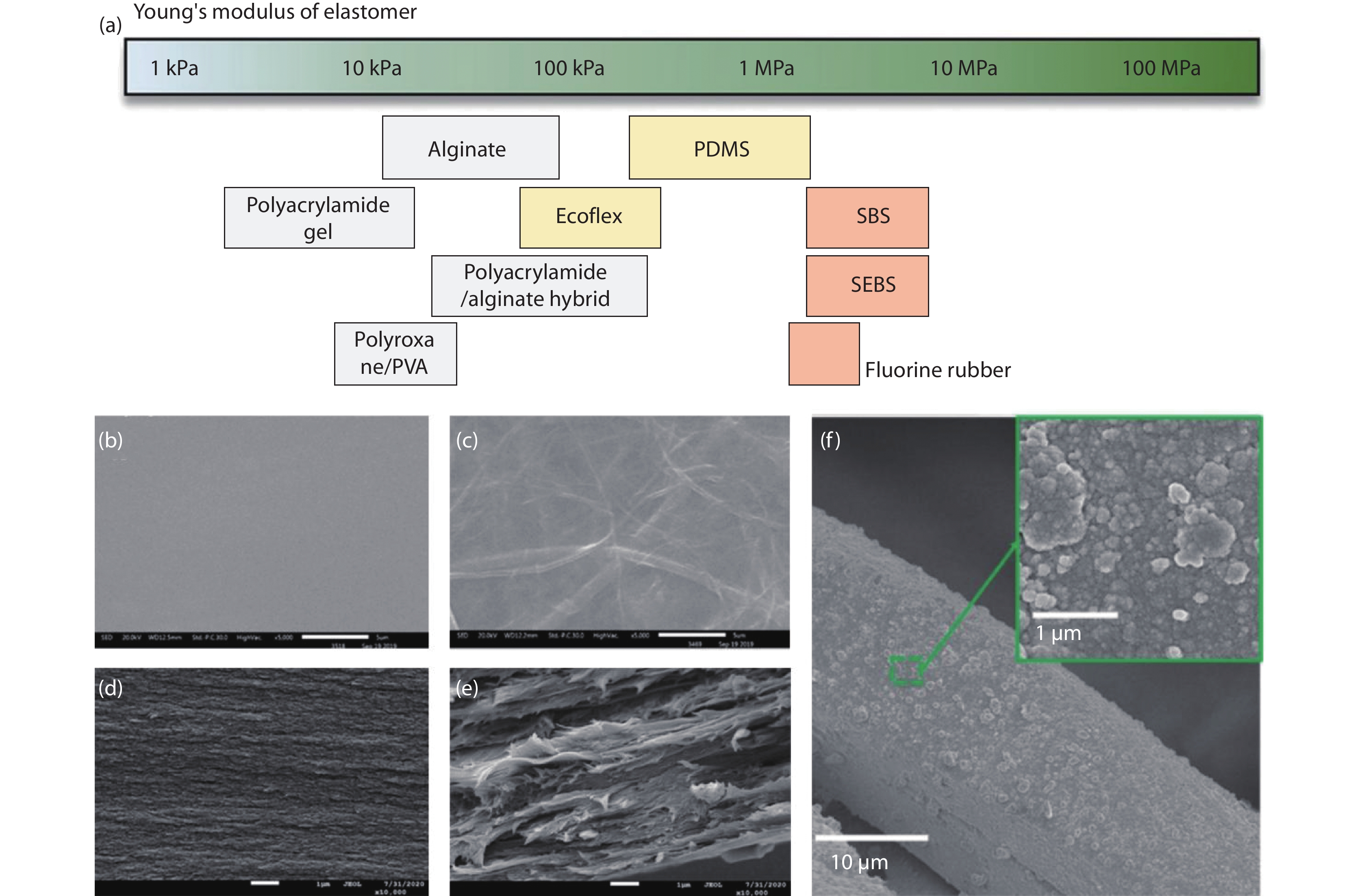

 DownLoad:
DownLoad: