| Citation: | Håvard Mølnås, Michael R. Scimeca, Ayaskanta Sahu. Harnessing high power factors with enhanced stability in heavy metal-free solution-processed thermoelectric copper sulfoselenide thin films[J]. Materials Lab, 2023, 2(1): 220040. doi: 10.54227/mlab.20220040 |
Harnessing high power factors with enhanced stability in heavy metal-free solution-processed thermoelectric copper sulfoselenide thin films
-
Abstract
Thermoelectric devices have the potential to recover waste heat from inefficient energy conversion processes. State-of-the-art thermoelectrics demonstrate low efficiency and incorporate materials containing rare and toxic elements. In this regard, p-type copper selenide (Cu2Se) has been identified as a promising and environmentally benign alternative. Unfortunately, the high diffusivity of liquid-like copper ions results in structural instability and performance degradation during operation, especially at moderate to high temperatures above 200 °C. Sulfur substitution has been utilized in melt-annealed samples to improve the stability of Cu2Se during operation, however this fabrication process is energy intensive and does not allow for use of flexible substrates. In this work, we report a solution-based direct thin film route to tune carrier concentration in copper sulfoselenide (Cu2-ySxSe1-x) thin films by controlling sulfur content and degree of copper saturation. We observe that improved thermoelectric performance through copper saturation in nominally copper-deficient Cu2-ySe films comes at a huge cost, with significantly reduced material stability due to enhanced copper migration resulting in severe degradation of the thermopower. Circumventing copper saturation, we show that controlled sulfur addition and tuning of annealing temperature has a synergistic effect, resulting in improved stability of the thermoelectric properties during continuous operation for mildly copper-deficient films while sustaining a high power factor of 800 μW m−1 K−2 at room temperature. Our results demonstrate a pathway for generating high performance solution processed thermoelectric devices with flexible form factors, and reinforce the case for Cu2-ySxSe1-x thin films as a heavy metal-free alternative for scavenging low grade waste heat.
-
Keywords:
- Copper selenide /
- Copper sulfoselenide /
- Stability /
- Thermoelectric
-
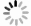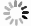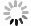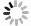
-
References
1. H. Ritchie and M. Roser (2021) - Energy. Published in Our World in Data. Online at: https://ourworldindata.org/energy 2. M. M. Kostic, Encyclopedia of Energy Engineering, Vol. II (Eds: S. Anwar), Taylor & Francis Group, USA, 2014, Ch. "Energy: Global and Historical Background". 3. G. J. Snyder and E. S. Toberer, Nat. Mater., 2008, 7, 105 4. Z. Zhang, K. Zhao, T.-R. Wei, P. Qiu, L. Chen and X. Shi, Energy Environ. Sci., 2020, 13, 3307 5. H. Liu, X. Shi, F. Xu, L. Zhang, W. Zhang, L. Chen, Q. Li, C. Uher, T. Day and G. J. Snyder, Nat. Mater., 2012, 11, 422 6. D. Yang, X. Su, J. Li, H. Bai, S. Wang, Z. Li, H. Tang, K. Tang, T. Luo, Y. Yan, J. Wu, J. Yang, Q. Zhang, C. Uher, M. G. Kanatzidis and X. Tang, Adv. Mater., 2020, 32, 2003730 7. W.-D. Liu, L. Yang and Z.-G. Chen, Nano Today, 2020, 35, 100938 8. G. Li, G. Song, N. Wang, F. Hu, Y. Wu, H. Du and J. Yuo, Surf. Interfaces, 2022, 28, 101651 9. T. Mao, P. Qiu, X. Du, P. Hu, K. Zhao, J. Xiao, X. Shi and L. Chen, Adv. Funct. Mater., 2020, 30, 1908315 10. G. Dennler, R. Chmielowski, S. Jacob, F. Capet, P. Roussel, S. Zastrow, K. Nielsch, I. Opahle and G. K. H. Madsen, Adv. Energy Mater., 2014, 4, 1301581 11. P. Qiu, M. T. Agne, Y. Liu, Y. Zhu, H. Chen, T. Mao, J. Yang, W. Zhang, S. M. Haile, W. G. Zeier, J. Janek, C. Uher, X. Shi, L. Chen and G. J. Snyder, Nat. Commun., 2018, 9, 2910 12. M. U. Farooq, S. Butt, K. Gao, X. Sun, X. Pang, S. U. Khan, W. Xu, F. Mohmed, A. Mahmood and N. Mahmood, Ceram. Int., 2016, 42, 8395 13. T. P. Bailey, S. Hui, H. Xie, A Olvera, P. F. P. Poudeu, X. Tang and C. Uher, J. Mater. Chem. A, 2016, 4, 17225 14. S. D. Kang, J.-H. Pöhls, U. Aydemir, P. Qiu, C. C. Stoumpos, R. Hanus, M. A. White, X. Shi, L. Chen, M. G. Kanatzidis and G. J. Snyder, Mater. Today Phys., 2017, 1, 7 15. K. Zhao, A. B. Blichfeld, H. Chen, Q. Song, T. Zhang, C. Zhu, D. Ren, R. Hanus, P. Qiu, B. B. Iversen, F. Xu, G. J. Snyder, X. Shi and L. Chen, Chem. Mater., 2017, 29, 6367 16. K. Zhao, A. B. Blichfeld, E. Eikeland, P. Qiu, D. Ren, B. B. Iversen, X. Shi and L. Chen, J. Mater. Chem. A, 2017, 5, 18148 17. K. Zhao, P. Qiu, Q. Song, A. B. Blichfeld, E. Eikeland, D. Ren, B. Ge, B. B. Iversen, X. Shi and L. Chen, Mater. Today Phys., 2017, 1, 14 18. S. Xiang, Y. Liang, M. Zhou and X. Zhang, J. Alloys Compd., 2022, 910, 164812 19. J. A. Perez-Taborda, L. Vera, O. Caballero-Calero, E. O. Lopez, J. J. Romero, D. G. Stroppa, F. Briones and M. Martin-Gonzalez, Adv. Mater. Technol., 2017, 2, 1700012 20. A. Wang, Y. Xue, J. Wang, X. Yang, J. Wang, Z. Li and S. Wang, Mater. Today Energy, 2022, 24, 100929 21. Y. Lu, Y. Ding, Y. Qiu, K. Cai, Q. Yao, H. Song, L. Tong, J. He and L. Chen, ACS Appl. Mater. Interfaces, 2019, 11, 12819 22. M. M. Mallick, A. Sarbajna, A. G. Rösch, L. Franke, H. Geßwein, Y. M. Eggeler and U. Lemmer, Appl. Mater. Today, 2022, 26, 101269 23. Z. Lin, C. Hollar, J. S. Kang, A. Yin, Y. Wang, H.-Y. Shiu, Y. Huang, Y. Hu, Y. Zhang and X. Duan, Adv. Mater., 2017, 29, 1606662 24. D. H. Webber and R. L. Brutchey, J. Am. Chem. Soc., 2013, 135, 15722 25. Y. Ma, P. B. Vartak, P. Nagaraj and R. Y. Wang, RSC Adv., 2016, 6, 99905 26. M. R. Scimeca, F. Yang, E. Zaia, N. Chen, P. Zhao, M. P. Gordon, J. D. Forster, Y.-S. Liu, J. Guo, J. J. Urban and A. Sahu, ACS Appl. Energy Mater., 2019, 2, 1517 27. N. Chen, M. R. Scimeca, S. J. Paul, S. B. Hafiz, Z. Yang, X. Liu, F. Yang, D.-K. Ko and A. Sahu, Nanoscale Adv., 2020, 2, 368 28. J. Yu, K. Zhao, P. Qiu, X. Shi and L. Chen, Ceram. Int., 2017, 43, 11142 29. L. Zhao, F. Y. Fei, J. Wang, F. Wang, C. Wang, J. Li, J. Wang, Z. Cheng, S. Dou and X. Wang, Sci. Rep., 2017, 7, 40436 30. S. C. Riha, R. D. Schaller, D. J. Gosztola, G. P. Wiederrecht and A. B. F. Martinson, J. Phys. Chem. Lett., 2014, 5, 4055 31. J.-H. Choi and Y.-K. Han, Curr. Appl. Phys., 2015, 15, 1417 32. J. D. Forster, J. J. Lynch, N. E. Coates, J. Liu, H. Jang, E. Zaia, M. P. Gordon, M. Szybowski, A. Sahu, D. G. Cahill and J. J. Urban, Sci. Rep., 2017, 7, 2765 33. L. Xue, C. Fang, W. Shen, M. Shen, W. Ji, Y. Zhang, Z. Zhang and X. Jia, Mod. Phys. Lett. B, 2020, 34, 2050006 34. Y. Yao, B.-P. Zhang, J. Pei, Y.-C. Liu and J.-F. Li, J. Mater. Chem. C, 2017, 5, 7845 35. Y.-H. Ji, Z.-H. Ge, Z. Li and J. Feng, J. Alloys Compd., 2016, 680, 273 -
Rights and permissions
This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.
-
Supplementary Information
Supporting_Information-MATLAB-2022-0040.R1 
Information
Article Metrics
-
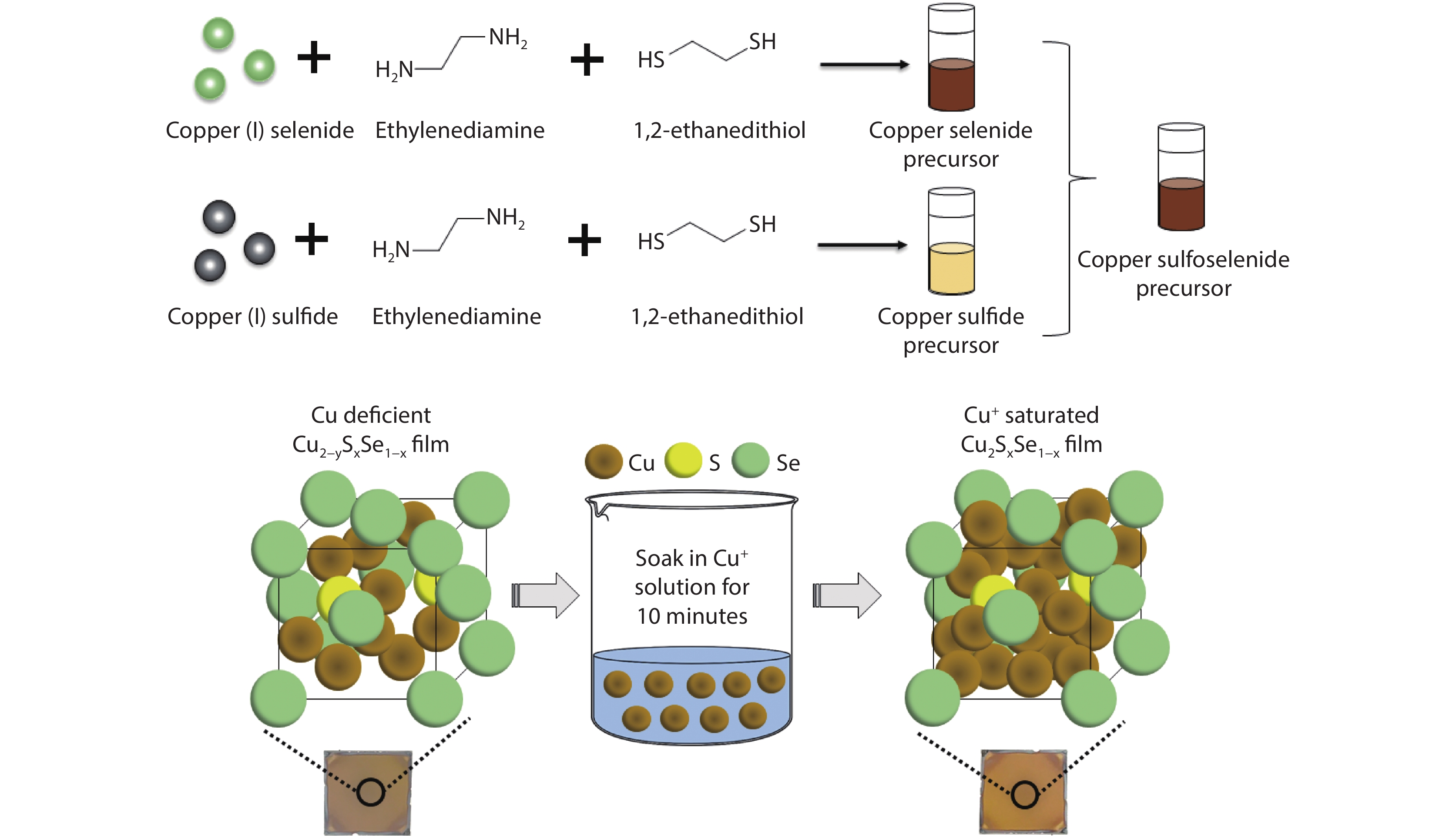
Figure 1.
Preparation of Cu2-ySxSe1-x precursor and Cu+ saturation of Cu2-ySxSe1-x films. [26] Copyright 2019, American Chemical Society.
-
Figure 1.
Room-temperature absorbance for unsoaked a and Cu soaked b Cu2-ySxSe1-x (x = 0.20 ± 0.019, 0.36 ± 0.005, 0.48 ± 0.032) films annealed at 350 °C. Partial and full quenching of the high wavelength plasmon peak is observed both by S substitution and Cu saturation, respectively.
-
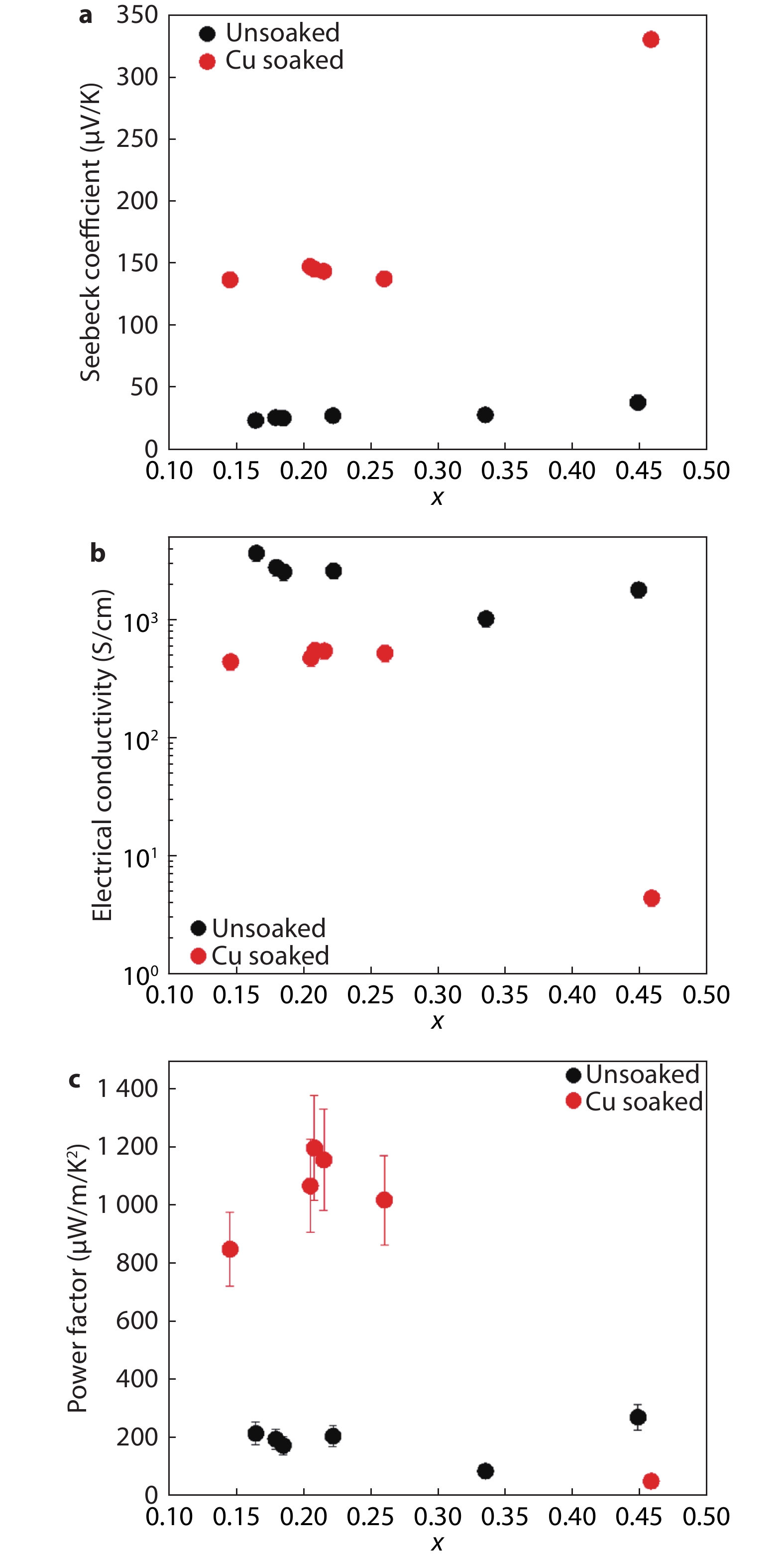
Figure 2.
Thermoelectric properties of unsoaked (black) and Cu soaked (red) Cu2-ySxSe1-x films annealed on hot plate at 350 °C as a function of S content, x. a, Seebeck coefficient as a function of S content before/after Cu soaking. A 5 – 7-fold increase in Seebeck coefficient is observed upon soaking as well as a slight increase upon increased S content. b, Electrical conductivity as a function of S content before/after Cu soaking. A significant decrease in electrical conductivity is observed after Cu soaking, and with increasing S content, especially for Cu soaked samples. Measurement uncertainties of electrical conductivity data were determined to ~15% and are captured within the data points. c, Power factor as a function of S content before/after 10 minutes soaking in 0.05 M Cu(I) in methanol. A 3 – 5-fold improvement of power factor is observed upon Cu soaking, except for x > 0.45. S substitution improves or sustains power factor up to x ~0.30 for soaked samples.
-
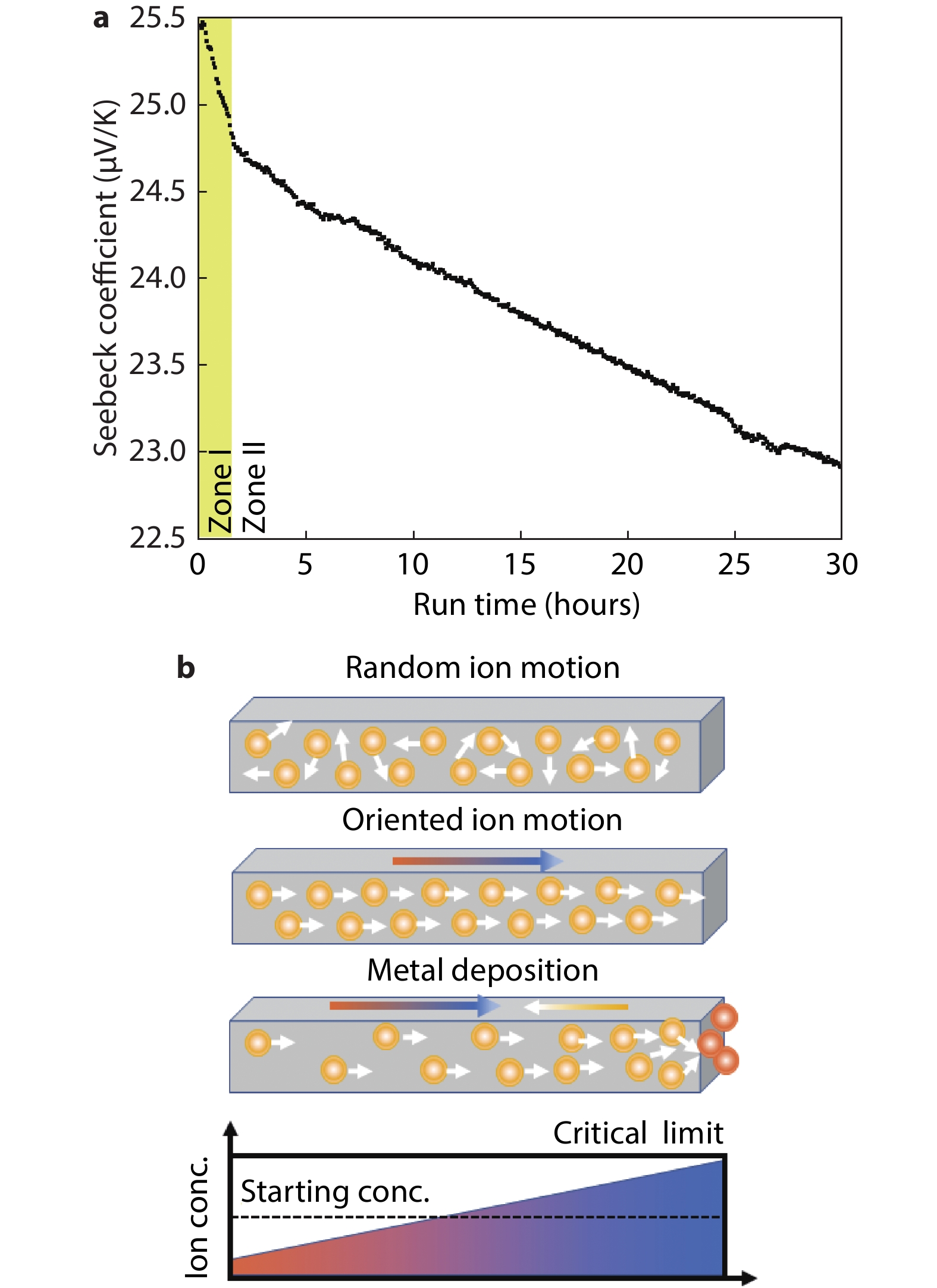
Figure 3.
a, Continuous Seebeck coefficient measurement of unsoaked Cu2-ySxSe1-x film annealed at 350 °C, without the extra addition of S (x = 0.16 ± 0.02 due to contribution from EDT solvent). The Seebeck coefficient decreases following two mechanisms - on a short time scale, ~2 hours (Zone I, yellow background), and at longer time scales (Zone II, white background). b, Schematic of random ion motion, oriented ion migration and metal deposition mechanism during thermoelectric device operation. Reproduced under terms of the CC-BY license.[11] Copyright 2018, Springer Nature.
-
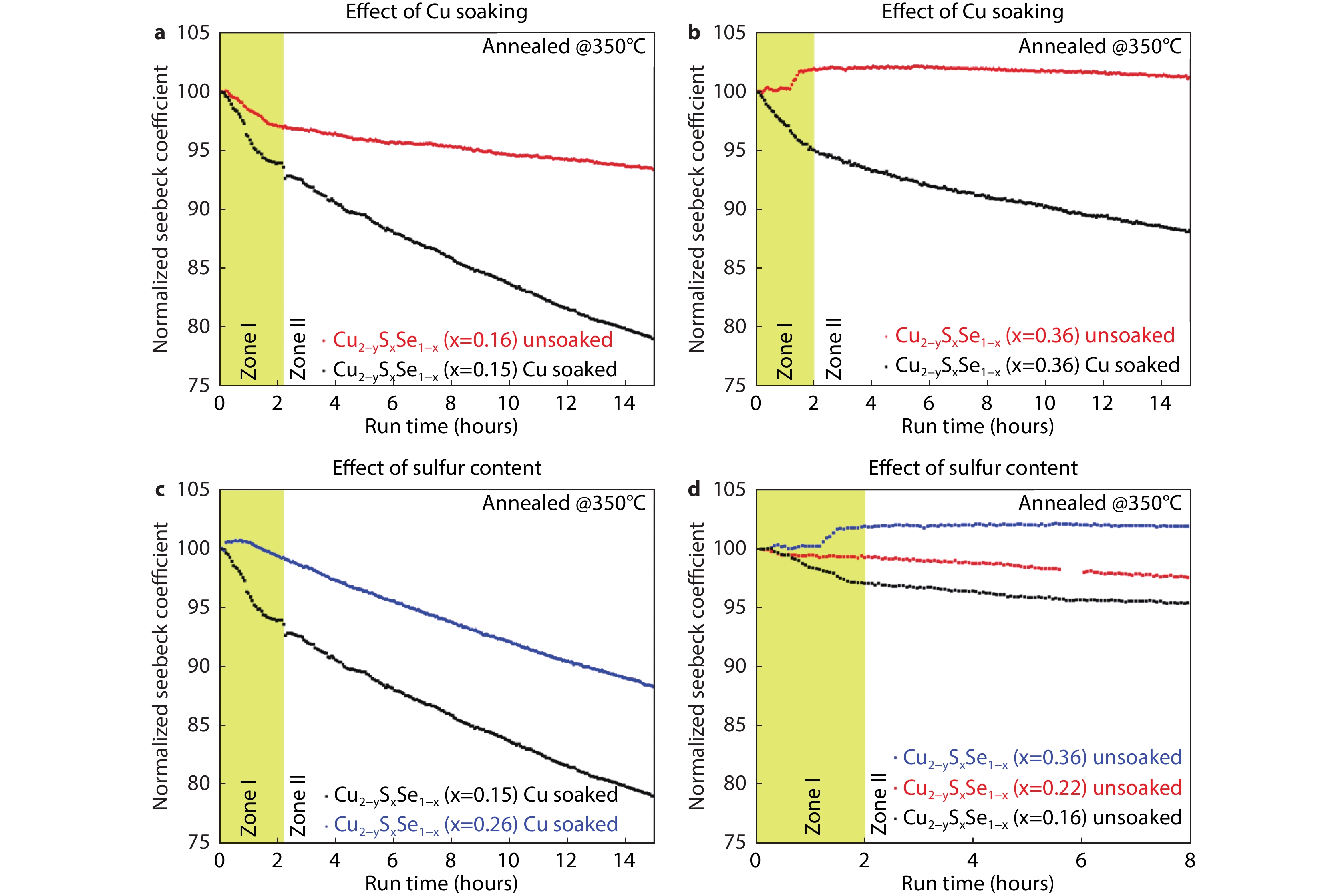
Figure 4.
Stability of Seebeck coefficient over time. The yellow box indicates the period of initial degradation mechanism, Zone I. a-b, Effect of Cu soaking for films with x = 0.15 − 0.16 and x = 0.36 annealed at 350 °C: Cu soaking decreases Seebeck coefficient stability. c-d, Effect of sulfur for unsoaked and Cu soaked films annealed at 350 °C: addition of S improves Seebeck coefficient stability.
-
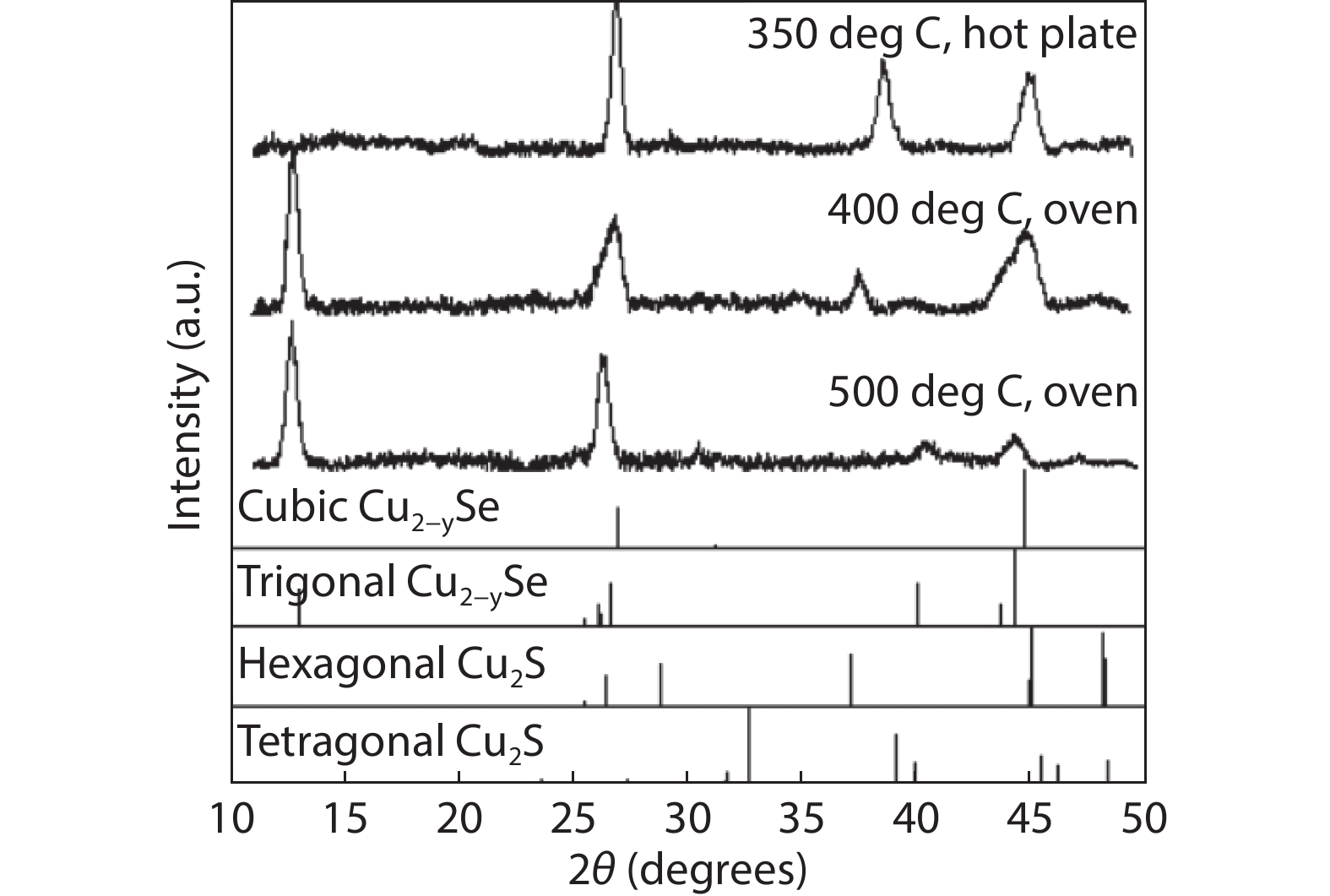
Figure 5.
Room-temperature X-ray diffraction patterns for unsaturated Cu2-ySxSe1-x (x = 0.16 ± 0.03) on glass annealed at different temperatures compared to reference spectra for cubic (ICSD
150758 ) and trigonal (ICSD243952 ) Cu2-ySe, and hexagonal (ICSD95397 ) and tetragonal (ICSD16550 ) Cu2-yS, respectively. The samples underwent thermoelectric characterization prior to XRD characterization. The trigonal Cu2-ySe phase appears with increasing annealing temperature. The origin of the peaks at 2ϴ ~38 is likely diffusion of metal ions from the gold contacts, however this is currently still under investigation. -
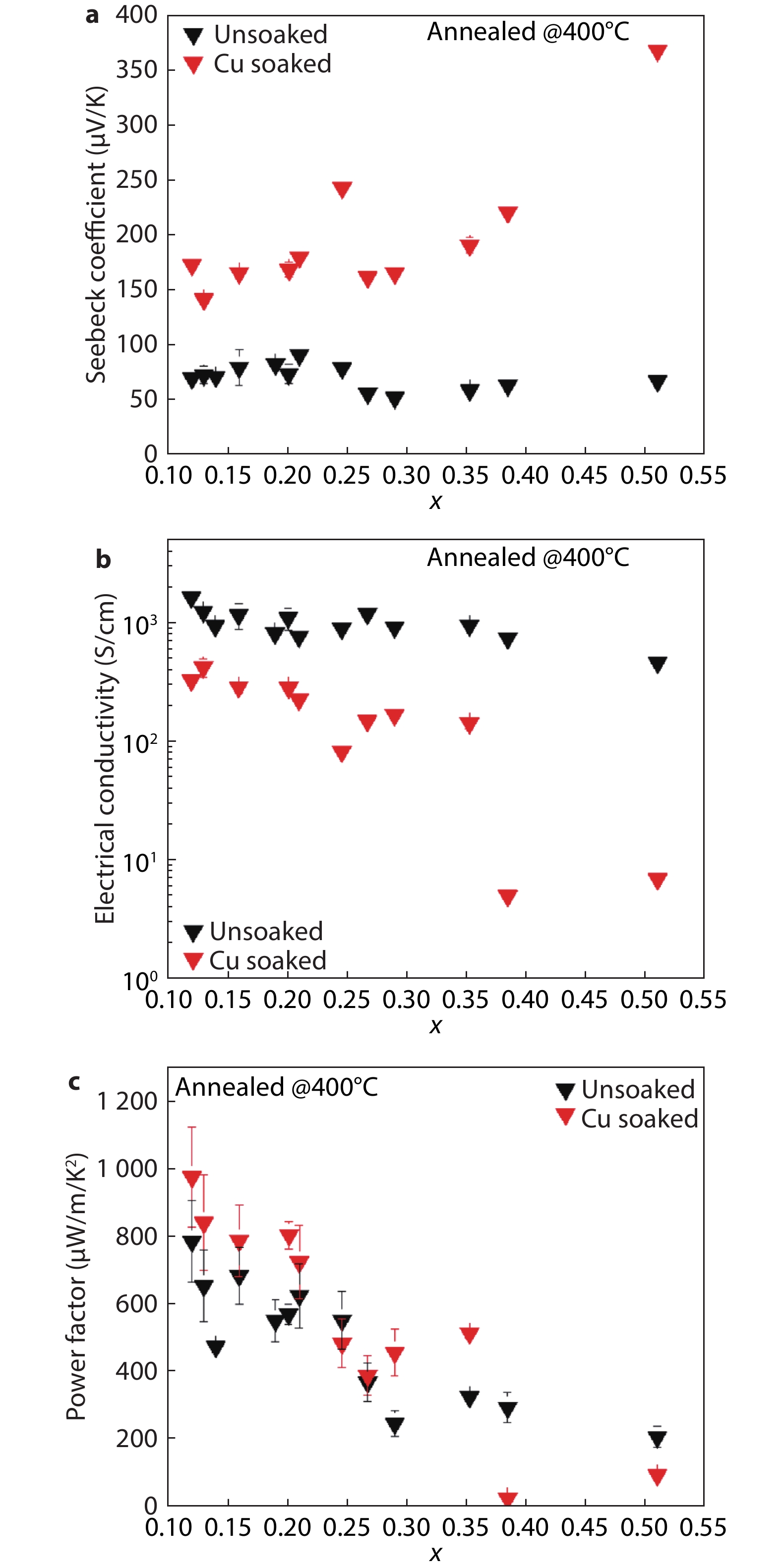
Figure 6.
Average thermoelectric properties of unsoaked (black) and Cu soaked (red) Cu2-ySxSe1-x films annealed in an oven at 400 °C as a function of S content, x. Standard deviations for y-values are shown as error bars where appropriate. Standard deviations for x-values are generally captured within the data points. The full data sets are shown in the SI (Figure S12). a, Seebeck coefficient. A 3-fold increase in Seebeck coefficient upon Cu soaking compared to the unsoaked sample is observed. The Seebeck coefficient of Cu soaked samples increases slightly with S content up to x~0.40. b, Electrical conductivity. A significant reduction in electrical conductivity is observed upon Cu soaking. Electrical conductivity decreases slightly with increasing S content. c, Power factor. The power factor increases 20 - 50% after 10 min soaking in 0.05 M Cu(I) in methanol compared to before soaking for x up to ~0.35. A decreasing trend in power factor with increasing x is observed for both unsoaked and Cu soaked samples. The S content in unsoaked samples is estimated based on the S content in the corresponding Cu soaked sample.
-
Figure 7.
Stability of Seebeck coefficient over time. The yellow box indicates the period of initial degradation mechanism, Zone I. a, Effect of annealing temperature for unsoaked films with x = 0.14 − 0.16: Increased Seebeck coefficient stability after annealing at 400 °C. b, Effect of operating temperature for unsoaked films with x = 0.14 − 0.16: Slightly reduced Seebeck coefficient stability at 50 °C compared to 23 °C.


 DownLoad:
DownLoad: