| Citation: | Dong Liu, Ting Tang, Li-Feng Zhu. Antiferroelectric capacitor for energy storage: a review from the development and perspective[J]. Materials Lab, 2024, 3(2): 230028. doi: 10.54227/mlab.20230028 |
Antiferroelectric capacitor for energy storage: a review from the development and perspective
-
Abstract
With the fast development of the power electronics, dielectric materials with large power densities, low loss, good temperature stability and fast charge and discharge rates are eagerly desired for the potential application in advanced pulsed power-storage system. Especially, antiferroelectric (AFE) capacitors which have been considered as a great potential for electric device applications with high energy density and output power are widely concentrated recently. To propel the development of dielectric capacitors marketization, in this view, we comprehensively summarized the development process of energy storage density and efficiency, improving strategy, raw materials cost and thermal steadily of the typical AFE capacitors, including Pb(Zr, Ti)O3, AgNbO3, (Bi, Na)TiO3, and NaNbO3 AFE systems. Moreover, the advantages and disadvantages of these AFE energy-storage ceramics are compared and discussed, which lay the foundation for the AFE energy storage capacitor early realization of marketization.
-
Keywords:
- Dielectric capacitor /
- antiferroelectric /
- raw materials cost /
- Energy storage
-
-
References
1. F. Z. Yao, Q. Yuan, Q. Wang, H. Wang, Nanoscale, 2020, 12, 17165 2. L. Qi, F. Z. Yao, Y. Liu, G. Z. Zhang, H. Wang, Q. Wang, The Annual Review of Materials Research, 2018, 48, 219 3. H. Palneedi, M. Peddigari, G. Hwang, D. Jeong, J. Ryu, Adv. Funct. Mater., 2018, 28, 1803665 4. G. Zhang, S. Zhang, Q. Wang, J. Materiomics., 2022, 8, 1287 5. P. Gao, Z. Liu, N. Zhang, H. Wu, A. Bokov, W. Ren, Z. Ye, Chem. Mater., 2019, 31, 979 6. X. K. Wei, C. L. Jia, H. C. Du, K. Roleder, J. Mayer, R. Dunin-Borkowski, Adv. Mater., 2020, 32, 1907208 7. J. Li, F. Li, Z. Xu, S. Zhang, Adv. Mater., 2018, 30, 1802155 8. P. Zhao, H. Wang, L. Wu, L. Chen, Z. Cai, L. Li, X. Wang, Adv. Energy Mater., 2019, 9, 1803048 9. N. Liu, R. Liang, Z. Zhou, X. Dong, J. Mater. Chem. C, 2018, 6, 10211 10. L. Zhu, X. Lei, L. Zhao, M, Hussain, G. Zhao, B. Zhang, Ceram. Inter., 2019, 45, 20266. 11. B. Luo, X. Wang, E. Tian, H. Song, H. Wang, L. Li, ACS Appl. Mater. Interfaces, 2017, 9, 19963 12. S. I. Shkuratov, J. Baird, V. G. Antipov, S. Zhang, J. B. Chase, Adv. Mater., 2019, 31, 1904819 13. L. Chen, N. Sun, Y. Li, Q. Zhang, L. Zhang, X. Hao, J. Am. Ceram. Soc., 2018, 101, 2313 14. Q. Zhang, Y. Dan, J. Chen, Y. Lu, T. Yang, Y. He, Ceram. Int., 2017, 43, 11428 15. H. Zhang, X. Chen, F. Cao, G. Wang, X. Dong, Z. Hu, T. Du, J. Am. Ceram. Soc., 2010, 93, 4015 16. Y. Wang, X. Hao, J. Yang, J. Xu, D. Zhao, J. Appl. Phys., 2012, 112, 034105 17. S. Jiang, L. Zhang, G. Zhang, S. Liu, J. Yi, X. Xiong, Y. Yu, J. He, Y. Zeng, Ceram. Int., 2013, 39, 5571 18. S. Chen, X. Wang, T. Yang, J. Wang, J. Electroceram., 2014, 32, 307 19. X. Yang, Y. Liu, C. He, H. Tailor, X. Long, J. Eur. Ceram. Soc., 2015, 35, 4173 20. X. Yang, C. He, Y. Liu, X. Li, Z. Wang, S. Han, S. Pan, X. Long, Ceram. Int., 2016, 42, 10472 21. L. Xu, C. He, X. Yang, Z. Wang, X. Li, H. M. Tailor, X. Long, J. Eur. Ceram. Soc., 2017, 37, 3329 22. H. Wang, Y. Liu, T. Yang, S. Zhang, Adv. Funct. Mater., 2019, 29, 1807321. 23. K. Huang, G. Ge, F. Yan, B. Shen, J. Zhai, Adv. Electron Mater., 2020, 6, 1901366. 24. X. Liu, T. Yang, W. Gong, J. Mater. Chem. C, 2021, 9, 12399. 25. L. Chen, J. Zhou, L. Xu, J. Ding, Z. Sun, Q. Bao, X. Hao, Chem. Eng. J., 2022, 447, 137367 26. X. Liu, T. Yang, B. Shen, L. Chen, ACS Appl. Energy Mater., 2023, 6, 1218 27. L. Zhang, S. Jiang, Y. Zeng, M. Fu, K. Han, Q. Li, Q. Wang, G. Zhang, Ceram. Int., 2014, 40, 5455 28. J. Xie, M. Yao, W. Gao, Z. Su, X. Yao, J. Eur. Ceram. Soc., 2019, 39, 1050 29. G. Ge, K. Huang, S. Wu, F. Yan, X. Li, B. Shen, J. Zhai, Energy Storage Mater., 2021, 35, 114 30. R. Xu, Q. Zhu, Z. Xu, Y. Feng, X. Wei, Appl. Phys. Lett., 2022, 120, 52904 31. X. Meng, Y. Zhao, J. Zhu, L. Zhu, Y. Li, X, Hao, J. Eur. Ceram. Soc., 2022, 42, 6493 32. X. Liu, J. Zhu, Y. Li, T. Yang, X. Hao, W. Gong, Chem. Eng. J., 2022, 446, 136729 33. L. Yang, X. Kong, F. Li, H. Hao, Z. Cheng, H. Liu, J.-F. Li, S. Zhang, Prog. Mater. Sci., 2019, 102, 72 34. Q. Xu, J. Xie, Z. He, L. Zhang, M. Cao, X. Huang, M. T. Lanagan, H. Hao, Z. Yao, H. Liu, J. Eur. Ceram. Soc., 2017, 37, 99 35. Z. Yu, Y. Liu, M. Shen, H. Qian, F. Li, Y. Lyu, Ceram. Int., 2017, 43, 7653 36. X. Zhou, H. Qi, Z. Yan, G. Xue, H. Luo, D. Zhang, ACS Appl. Mater. Interfaces, 2019, 11, 43107 37. X. Qiao, F. Zhang, D. Wu, B. Chen, X. Zhao, Z. Peng, X. Ren, P. Liang, X. Chao, Z. Yang, Chem. Eng. J., 2020, 388, 124158 38. L. Zhang, R. Jing, Y. Huang, Q. Hu, D. O. AliKin, V. Y. Shur, J. Gao, X. Wei, L. Zhang, G. Liu, Y. Yan, L. Jin, J. Materiomics., 2022, 8, 527 39. Y. Zhang, A. Xie, J. Fu, X. Jiang, T. Li, C. Zhou, R. Zuo, ACS Appl. Mater. Interfaces, 2022, 14, 40043 40. R. Kang, Z. Wang, M. Wu, S. Cheng, S. Mi, Y. Hu, L. Zhang, D. Wang, X. Lou, Nano Energy, 2023, 112, 108477 41. Y. Pu, M. Yao, L. Zhang, P. Jing, J. Alloy Compd., 2016, 687, 689 42. B. Hu, H. Q. Fan, N. Li, S. Gao, Z. J. Yao, Q. Li, Ceram. Int., 2018, 44, 10968 43. H. Qi, R. Zuo, J. Mater. Chem. A, 2019, 7, 3971 44. T. Wei, K. Liu, P. Fan, D. Lu, B. Ye, C. Zhou, H. Yang, H. Tan, D. Salamon, B. Nan, H. Zhang, Ceram. Int., 2021, 47, 3713 45. J. Li, Z. Shen, X. Chen, S. Yang, W. Zhou, M. Wang, L. Wang, Q. Kou, Y. Liu, Q. Li, Z. Xu, Y. Chang, Nat. Mater., 2020, 19, 999 46. D. Fu, M. Endo, H. Taniguchi, T. Taniyama, M. Itoh, Appl. Phys. Lett., 2007, 90, 252907 47. L. Zhao, J. Gao, Q. Liu, S. Zhang, J.-F. Li, ACS Appl. Mater. Interfaces, 2018, 10, 819 48. N. Luo, K. Han, L. Liu, B. Peng, X. Wang, C. Hu, H. Zhou, Q. Feng, X. Chen, Y. Wei, J. Am. Ceram. Soc., 2019, 102, 4640 49. H. Pan, J. Ma, J. Ma, Q. Zhang, X. Liu, B. Guan, L. Gu, X. Zhang, Y.-J. Zhang, L. Li, Y. Shen, Y.-H. Lin, C.-W, Nan, Nat. Commun., 2018, 9, 1813 50. H. Pan, F. Li, Y. Liu, Q. Zhang, M. Wang, S. Lan, Y. Zheng, J. Ma, L. Gu, Y. Shen, P. Yu, S. Zhang, L.-Q. Chen, Y.-H. Lin, C.-W, Nan, Science, 2019, 365, 578 51. L. Zhao, Q. Liu, S. Zhang, J.-F. Li, J. Mater. Chem. C, 2016, 4, 8380 52. L. Zhao, Q. Liu, J. Gao, S. Zhang, J.-F. Li, Adv. Mater., 2017, 29, 1701824 53. C. Xu, Z. Fu, Z. Liu, L. Wang, S. Yan, X. Chen, F. Cao, X. Dong, G. Wang, ACS Sustain Chem. Eng., 2018, 6, 16151 54. N. Luo, K. Han, F. Zhuo, C. Xu, G. Zhang, L. Liu, X. Chen, C. Hu, H. Zhou, Y. Wei, J. Mater. Chem. A, 2019, 7, 14118 55. N. Luo, K. Han, M. J. Cabral, X. Liao, S. Zhang, C. Liao, G. Zhang, X. Chen, Q. Feng, J.-F. Li, Y. Wei, Nat. Commun., 2020, 11, 4824 56. W. Chao, J. Gao, T. Yang, Y. Li, J. Eur. Ceram. Soc., 2021, 41, 7670 57. Y. Xu, Z. Yang, K. Xu, J. Tian, D. Zhang, M. Zhan, H. Tian, X. Cai, B. Zhang, Y. Yan, L. Guo, G. Wang, L. Lin, J. Fan, T. Wang, Y. Tian, J. Alloy. Compod., 2022, 913, 165313 58. L. He, Y. Yang, C. Liu, Y. Ji, X. Lou, L. Zhang, X. Ren, Acta Materialia., 2023, 249, 118826 59. J. Gao, Q. Liu, J. Dong, X. Wang, S. Zhang, J.-F. Li, ACS Appl. Mater. Interfaces, 2020, 12, 6097 60. K. Han, N. Luo, Y. Jing, X. Wang, B. Peng, L. Liu, C. Hu, H. Zhou, Y. Wei, X. Chen, Q. Feng, Ceram. Int., 2019, 45, 5559 61. S. Mao, N. Luo, K. Han, Q. Feng, X. Chen, B. Peng, L. Liu, C. Hu, H. Zhou, F. Toyohisa, Y. Wei, J. Mater. Sci: Mater. El., 2020, 31, 7731 62. S. Li, H. Nie, G. Wang, C. Xu, N. Liu, M. Zhou, F. Cao, X. Dong, J. Mater. Chem. C, 2019, 7, 1551 63. L.-F. Zhu, S. Deng, L. Zhao, G. Li, Q. Wang, L. Li, Y. Yan, H. Qi, B.-P. Zhang, J. Chen, J.-F. Li, Nat. Commun., 2023, 14, 1166 64. R. H. Dungan, R. D, J. Am. Ceram. Soc., 1964, 47, 73 65. A. V. Ulinzheyev, O. E. Fesenko, V. G. Smotrakov, Ferroelectrics Lett., 1990, 12, 17 66. A. V. Ulinzheyev, A. V. Leiderman, V. G. Smotrakov, V. Y. Topolov, O. E. Fesenko, Phys. solid state, 1997, 39, 972 67. V. A. Shuvaeva, M. Y. Antipin, R. S. V. Lindeman, O. E. Fesenko, V. G. Smotrakov, Y. T. Struchkov, Ferroelectrics, 1993, 141, 307 68. T. Arioka, H. Taniguchi, M. Itoh, K. Oka, R. Wang, D. Fu, Ferroelectrics, 2010, 401, 51 69. H. Shimizu, K. Kobayashi, Y. Mizuno, Clive A. Randall, J. Am. Ceram. Soc., 2014, 97, 1791 70. Y. Xu, W. Hong, Y. Feng, X. Tan, Appl. Phys. Lett., 2014, 104, 52903 71. D. Fu, T. Arioka, H. Taniguchi, T. Taniyama, M. Itoh, Appl. Phys. Lett., 2011, 99, 012904 72. H. Shimizu, H. Guo, S. E. Reyes-Lillo, Y. Mizuno, K. M. Rabe, C. A. Randall, Dalton Trans., 2015, 44, 10763 73. H. Guo, H. Shimizu, Y. Mizuno, C. A. Randall, J. Appl. Phys., 2015, 118, 054102 74. L. Gao, H. Guo, S. Zhang, C. A. Randall, J. Appl. Phys., 2016, 120, 204102 75. J. Ye, G. Wang, X. Chen, F. Cao, X. Dong, Appl. Phys. Lett., 2019, 114, 122901 76. Z. Liu, J. Lu, Y. Mao, P. Ren, H. Fan, J. Eur. Ceram. Soc., 2018, 38, 4939 77. H. Qi, R. Zuo, A. Xie, A. Tian, J. Fu, Y. Zhang, S. Zhang, Adv. Funct. Mater., 2019, 29, 1903877 78. A. Xie, H. Qi, R. Zuo, ACS Appl. Mater. Interfaces, 2020, 12, 19467 79. J. Shi, X. Chen, X. Li, J. Sun, C. Sun, F. Pang, H. Zhou, J. Mater. Chem. C, 2020, 8, 3784 80. R. Jing, L. Jin, Y. Tian, Y. Huang, Y. Lan, J. Xu, Q. Hu, H. Du, X. Wei, D. Guo, J. Gao, F. Gao, Ceram. Int., 2019, 45, 21175 81. Y. Fan, Z. Zhou, R. Liang, X. Dong, J. Eur. Ceram. Soc., 2019, 39, 4770 82. H. Qi, R. Zuo, A. Xie, X. Fu, D. Zhang, J. Eur. Ceram. Soc., 2019, 39, 3703 83. M. Zhou, R. Liang, Z. Zhou, S. Yan, X. Dong, ACS Sustain. Chem. Eng., 2018, 6, 12755 84. M. Zhou, R. Liang, Z. Zhou, S. Yan, X. Dong, J. Mater. Chem. A, 2018, 6, 17896 85. M. Zhou, R. Liang, Z. Zhou, X. Dong, Sustain Energ. Fuels, 2020, 4, 1225 86. N. Qu, H. Du, X. Hao, J. Mater. Chem. C, 2019, 7, 7993 87. R. Shi, Y. Pu, W. Wang, X. Guo, J. Li, M. Yang, S. Zhou, J. Alloy. Compd., 2020, 815, 152356 88. A. Tian, R. Zuo, H. Qi, M. Shi, J. Mater. Chem. A, 2020, 8, 8352 89. J. Jiang, X. Meng, L. Li, J. Zhang, S. Guo, J. Wang, X. Hao, H. Zhu, S. Zhang, Chem. Eng. J., 2021, 422, 130130 90. T. Pan, J. Zhang, D. Che, Z. Wang, J. Wang, J. Wang, Y. Wang, Appl. Phys. Lett., 2023, 122, 72902 91. P. Qiao, Y. Zhang, X. Chen, M. Zhou, G. Wang, X. Dong, J. Alloy. Compd., 2019, 780, 581 92. H. Yuan, X. Fan, Z. Zheng, M. Zhao, L. Zhao, K. Zhu, J. Wang, Chem. Eng. J., 2023, 456, 141023 93. S. Gao, Y. Huang, Y. Jiang, M. Shen, H. Huang, S. Jiang, Y. He, Q. Zhang, Acta Materialia, 2023, 246, 118730. 94. M. Wang, Q. Feng, C. Luo, Y. Lan, C. Yuan, N. Luo, C. Zhou, T. Fujita, J. Xu, G. Chen, Y. Wei, ACS Appl. Mater. Interfaces, 2021, 13, 51218 95. J. Liu, P. Li, C. Li, W. Bai, S. Wu, P. Zheng, J. Zhang, J. Zhai, ACS Appl. Mater. Interfaces, 2022, 14, 17662 96. A. Xie, J. Fu, R. Zuo, C. Zhou, Z. Qiao, T. Li, S. Zhang, Chem. Eng. J., 2022, 429, 132534 97. W. Chao, L. Tian, T. Yang, Y. Li, Z. Liu, Chem. Eng. J., 2022, 433, 133814 98. Y. Zhou, S. Gao, J. Huang, M. Shen, S. Jiang, Y. He, Q. Zhang, J. Materiomics, 2023, 9, 410. 99. D. Feng, H. Du, H. Ran, T. Lu, S. Xia, L. Xu, Z. Wang, C. Ma, J. Solid State Chem., 2022, 310, 123081 100. Y. Xu, Y. Guo, Q. Liu, Y. Yin, J. Bai, L. Lin, J. Tian, Y. Tian, J. Alloy. Compd., 2020, 821, 153260 101. J. Ai, X. Chen, L. Luo, R. Zheng, L. Xu, Ceram. Int., 2022, 48, 23630 102. S. Li, T. Hu, H. Nie, Z. Fu, C. Xu, F. Xu, G. Wang, X. Dong, Energy Storage Mater., 2021, 34, 417 103. L.-F. Zhu, L. Zhao, Y. Yan, H. Leng, X. Li, L.-Q. Cheng, X. Xiong, S. Priya, J. Mater. Chem. A, 2021, 9, 9655 104. Z. Lu, W. Bao, G. Wang, S.-K. Sun, L. Li, J. Lei, H. Yang, H. Ji, A. Feteira, D. Li, F. Xu, A. K. Kleppe, D. Wang, S.-Y. Liu, I. M. Reaney, Nano Energy, 2021, 79, 105423 105. J. Gao, Y. Zhang, L. Zhao, K.-Y. Lee, Q. Liu, A. Studer, M. Hinterstein, S. Zhang, J.-F. Li, J. Mater. Chem. A, 2019, 7, 2225 106. M. Shang, P. Ren, D. Ren, X. Wang, X. Lu, F. Yan, G. Zhao, Mater. Res. Bull., 2023, 157, 112008 107. J. Li, L. Jin, Y. Tian, C. Chen, Y. Lan, Q. Hu, C. Li, X. Wei, H. Yan, J. Materiomics, 2022, 8, 266 108. M. Zhao, J. Wang, H. Yuan, Z. Zheng, L. Zhao, J. Materiomics, 2023, 9, 19 109. P. Shi, X. Wang, X. Lou, C. Zhou, Q. Liu, L. He, S. Yang, X. Zhang, J. Alloy. Compd., 2021, 877, 160162 110. B. Li, Z. Yan, X. Zhou, H. Qi, V. Koval, X. Luo, H Luo, H. Yan, D. Zhang, ACS Appl. Mater. Interfaces, 2023, 15, 4246 111. L. Ma, Z. Chen, Z. Che, Q. Feng, Z. Cen, F. Toyohisa, Y. Wei, C. Hu, L. Liu, N. Luo, J. Eur. Ceram. Soc., 2022, 42, 2204 112. K. Han, N. Luo, S. Mao, F. Zhuo, L. Liu, B. Peng, X. Chen, C. Hu, H. Zhou, Y. Wei, J. Mater. Chem. A, 2019, 7, 26293 113. L. Ma, Z. Che, C. Xu, Z. Cen, Q. Feng, X. Chen, F. Toyohisa, J.-F. Li, S. Zhang, N. Luo, J. Eur. Ceram. Soc., 2023, 43, 3228 114. P. Ge, X. Tang, K. Meng, X.-X. Huang, Q.-X. Liu, Y.-P. Jiang, W.-P. Gong, T. Wang, Mater. Today Phys., 2022, 24, 100681 115. K. Huang, G. Ge, H. Bai, F. Yan, X. He, Y. Shi, B. shen, J. Zhai, J. Eur. Ceram. Soc., 2021, 41, 2450 116. P. Ge, X. Tang, K. Meng, X.-X. Huang, S.-F. Li, Q.-X. Liu, Y.-P. Jiang, Chem. Eng. J., 2022, 429, 132540 117. X. Meng, Y. Zhao, Y. Li, X. Hao, J. Am. Ceram. Soc., 2021, 104, 2170 118. G. Ge, H. Bai, Y. Shi, C. Shi, X. He, J. He, B. Shen, J. Zhai, X. Chou, J. Mater. Chem. A, 2021, 9, 11291 119. Y. Wu, Y. Fan, N. Liu, P. Peng, M. Zhou, S. Yan, F. Cao, X. Dong, G. Wang, J. Mater. Chem. C, 2019, 7, 6222 120. P. Ren, Z. Liu, X. Wang, Z. Duan, Y. Wan, F. Yan, G. Zhao, J. Alloy. Compd., 2018, 742, 683 121. X. Wu, H. Liu, J. Chen, J. Mater. Res., 2021, 36, 1153 122. Y. Pu, L. Zhang, Y. Cui, M. Chen, ACS Sustain. Chem. Eng., 2018, 6, 6102 123. D. He, Y. Wang, S. Song, S. Liu, Y. Luo, Y. Deng, Compos. Sci. Technol., 2017, 151, 25 124. Y. Shen, L. Wu, J. Zhao, J. Liu, L. Tang, X. Chen, H. Li, Z. Su, Y. Zhang, J. Zhai, Z. Pan, Chem. Eng. J., 2022, 439, 135762 125. Q. Wang, B. Xie, Q. Zheng, M. A. Marwat, Z. Liu, P. Mao, S. Jiang, H. Zhang, Chem. Eng. J., 2023, 452, 139422 126. R. Kang, Z. Wang, W. Yang, X. Zhu, L. He, Y. Gao, J. Zhao, P. Shi, Y. Zhao, P. Mao, Y. Hu, L. Zhang, X. Lou, Chem. Eng. J., 2022, 446, 137105 127. B. Laubacker, K. Wang, M. Wetherington, N. Wonderling, J. V. Badding, S. E. Mohney, J. Mater Sci: Mater. Ele., 2023, 34, 741 -
Rights and permissions
This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.
Information
Article Metrics
-
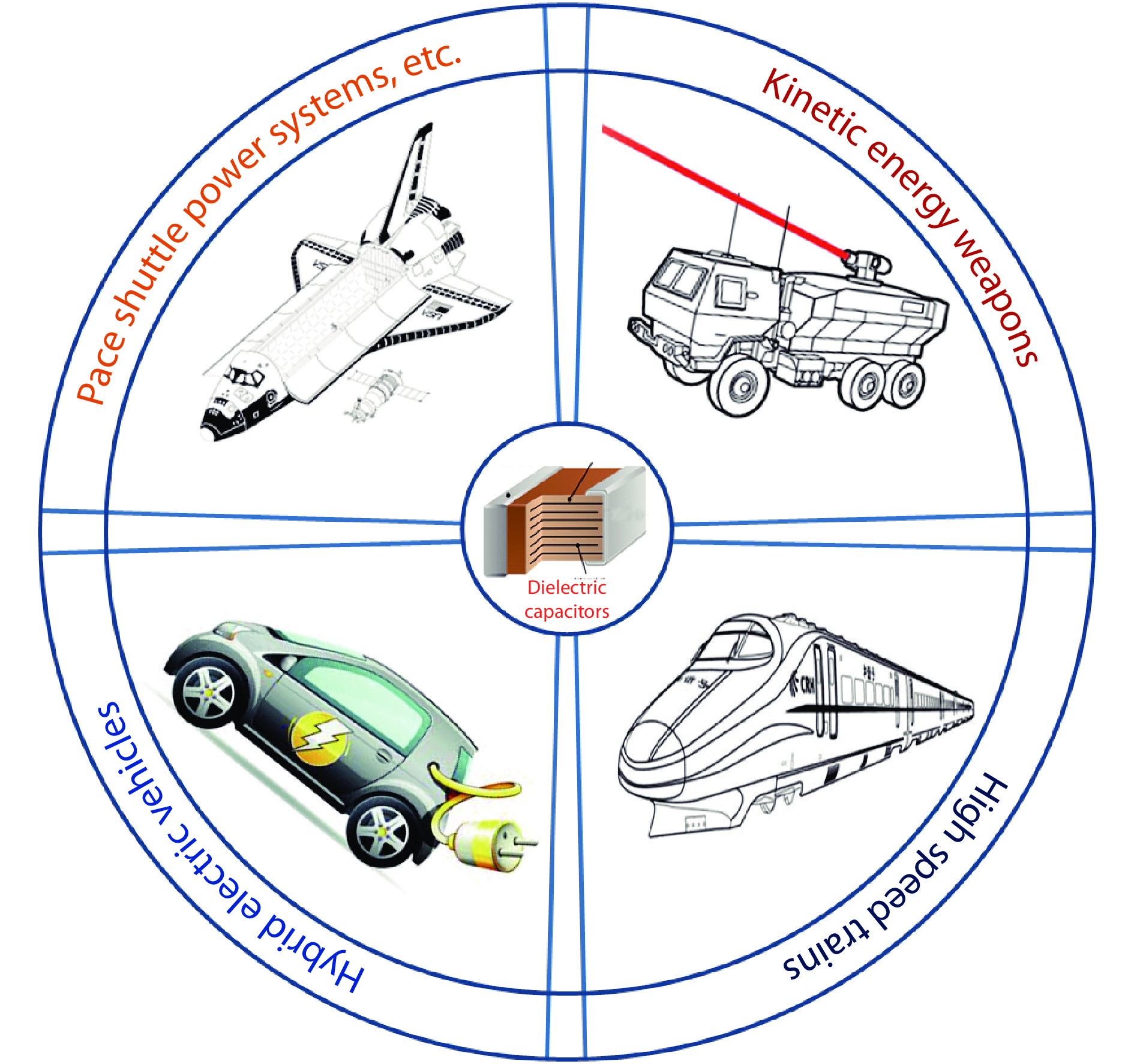
Figure 1.
The application of dielectric energy storage materials in pulsed-discharge and power conditioning electronic devices.
-
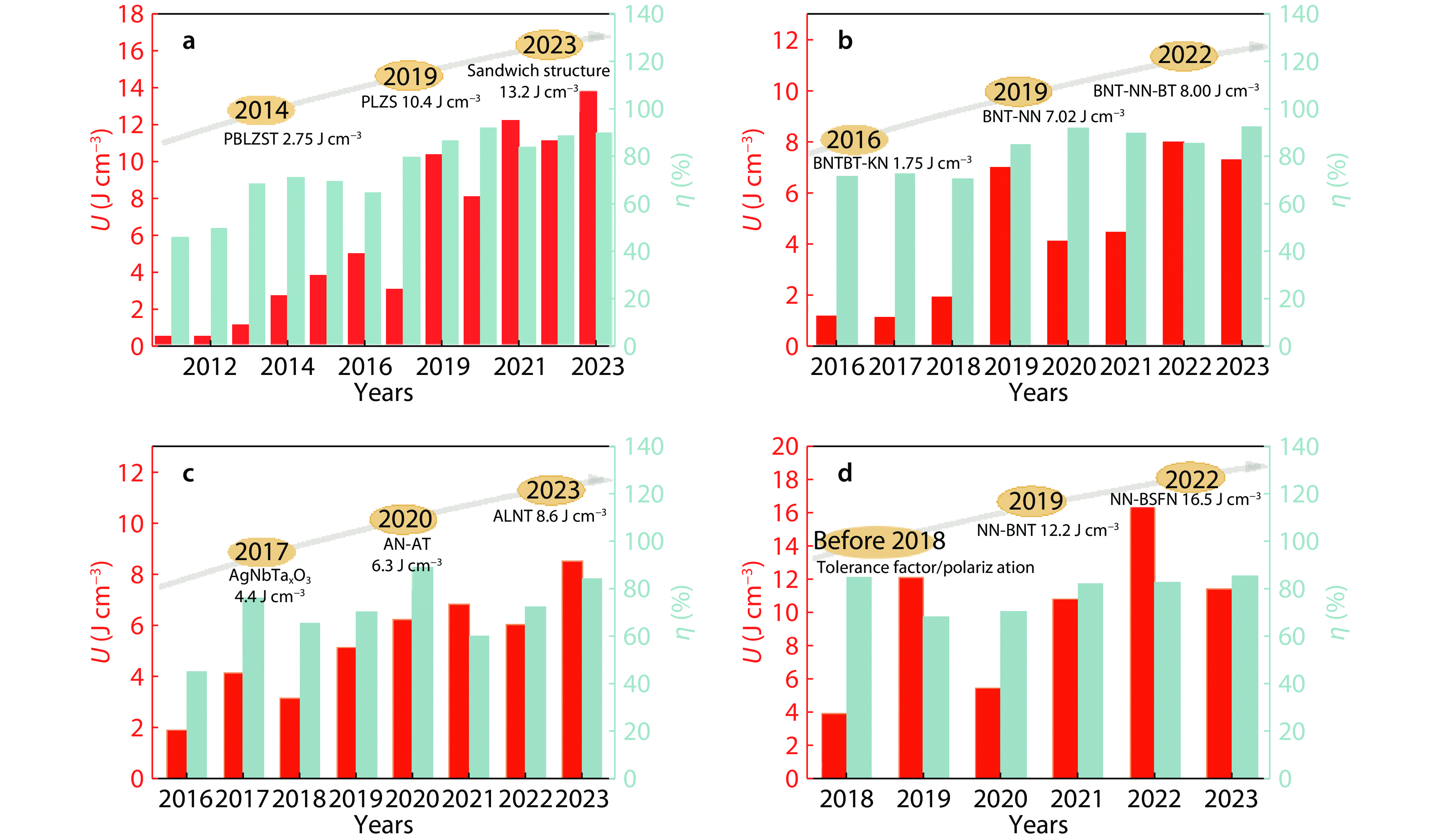
Figure 2.
Comparison of energy-storage properties among four typical anti-ferroelectric ceramics in recent 10 years.
-
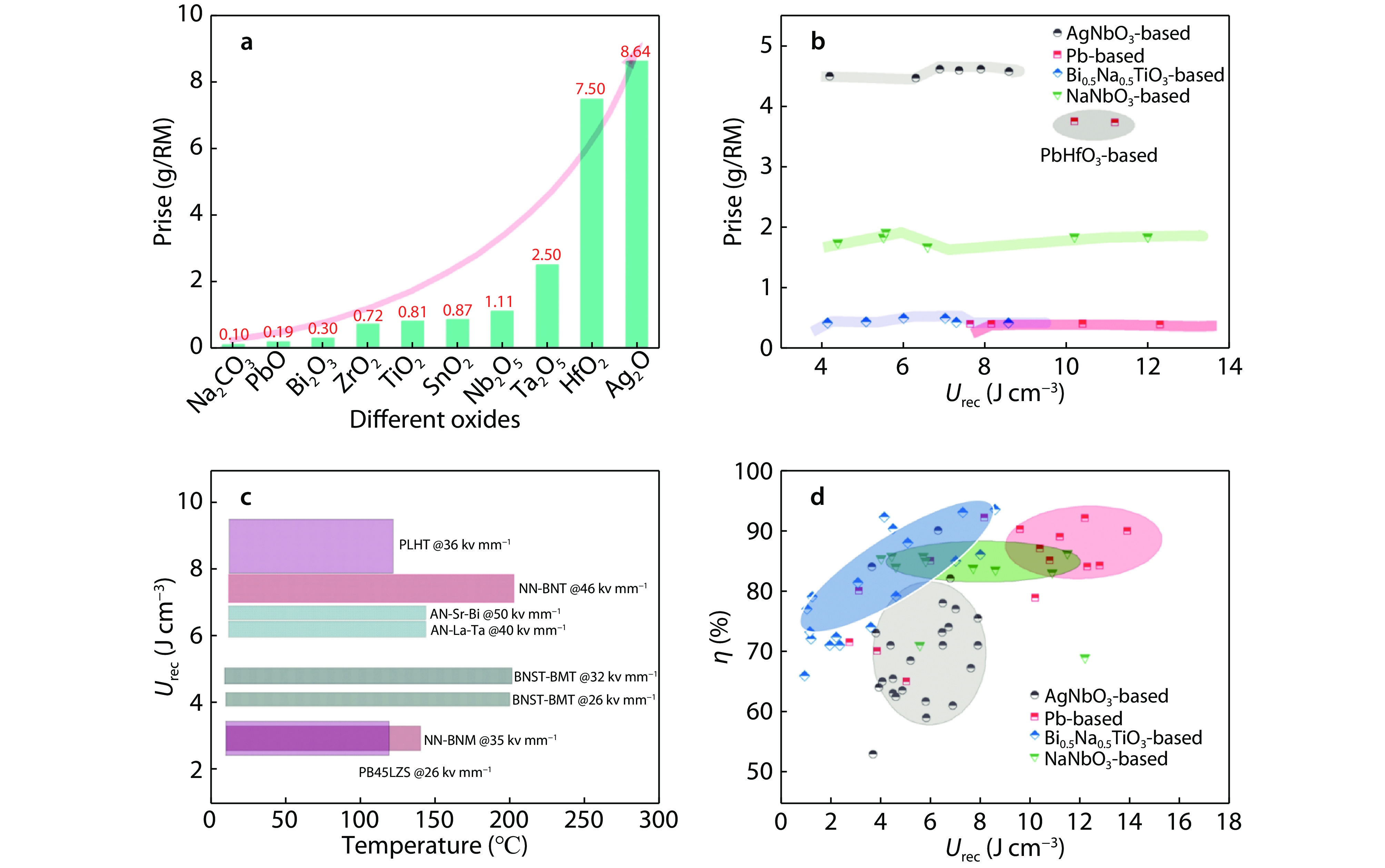
Figure 3.
a The prices of commonly used oxides. Comparison of b raw material price, c temperature stability and d energy storage performance of four typical antiferroelectric ceramics reported recently.

 Dong Liu, obtained his B.A. at Chengdu University of Information and Technology in 2017. He also received his M.D. at Guangdong University of Technology in 2020. Now he was a senior doctor in University of Science & Technology Beijing. He is mainly engaged in high power dielectric energy storage materials and texture piezoelectric materials.
Dong Liu, obtained his B.A. at Chengdu University of Information and Technology in 2017. He also received his M.D. at Guangdong University of Technology in 2020. Now he was a senior doctor in University of Science & Technology Beijing. He is mainly engaged in high power dielectric energy storage materials and texture piezoelectric materials.  Li-feng Zhu, received his PhD in University of Science & Technology Beijing in 2015. He was a visiting scholar in the Department of Materials Science and Engineering at Penn State University from September 2019 to October 2020. He is mainly engaged in the basic research of piezoelectric materials and devices and high power dielectric energy storage materials.
Li-feng Zhu, received his PhD in University of Science & Technology Beijing in 2015. He was a visiting scholar in the Department of Materials Science and Engineering at Penn State University from September 2019 to October 2020. He is mainly engaged in the basic research of piezoelectric materials and devices and high power dielectric energy storage materials. 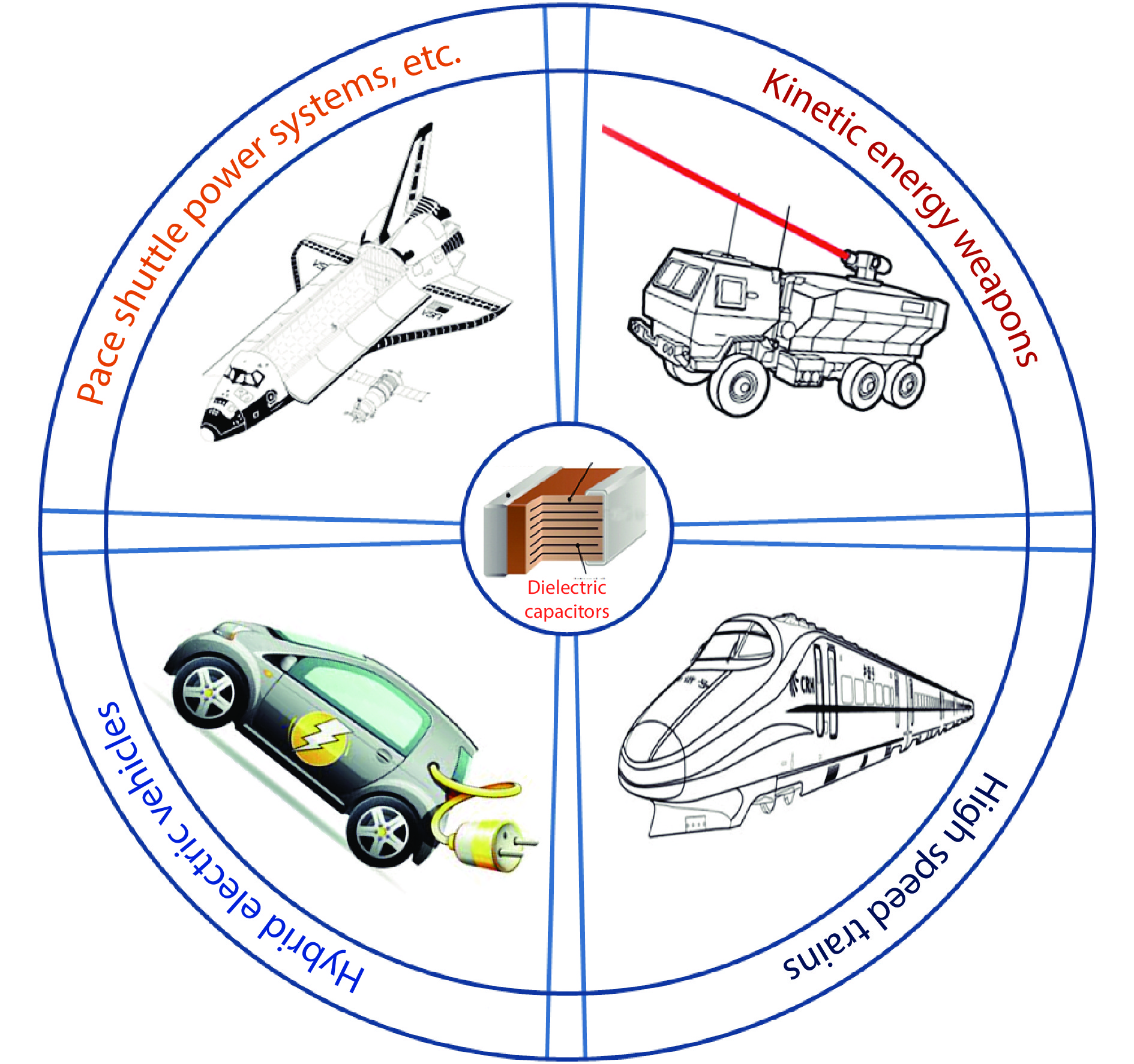

 DownLoad:
DownLoad: