| Citation: | Xinyue Han, Yuyang Qiu, Bin Zhang, Yunzhu Huang, Yusong Wang, Pan Feng, Xin Gao, Qian Zhu, Menghui Liao, Yangnan Hu, Renjie Chai. Advances in Organ-on-a-chip Technologies for Biomedical Research and Applications[J]. Materials Lab, 2025, 4(1): 240006. doi: 10.54227/mlab.20240006 |
Advances in Organ-on-a-chip Technologies for Biomedical Research and Applications
-
Abstract
Recent breakthroughs in micro-nano fabrication and tissue engineering have accelerated the evolution of organoids and organ-on-chips (OOCs), offering a novel
in vitro modeling strategy for human organ physiology. OOCs leverage microfluidic systems to replicate organ-specific microenvironments, providing a dynamic and configurable platform that simulates human biology for drug discovery and disease modeling. These technologies overcome the limitations of traditionalin vitro systems and animal models, with organoids enhancing disease progression insights and OOCs enabling reproducible, high-throughput tissue functionality assessment. Despite the establishment of various disease models and the successful commercial transition of certain OOC platforms, high costs and research limitations underscore the need for continued technological innovation and standardization. This review examines the current capabilities and challenges of OOCs in drug screening, disease modeling and personalized medicine, emphasizing their potential to refine evaluation processes with greater precision.-
Keywords:
- drug screening /
- disease modeling /
- personalized medicine.
-
-
References
1. Z. Li, D. Yu, C. Zhou, F. Wang, K. Lu, Y. Liu, J. Xu, L. Xuan, X. Wang, Biomater. Transl., 2024, 5, 21 2. L. H. Chi, A. D. Burrows, R. L. Anderson, Drug Discov. Today, 2022, 27, 257 3. E. V. Romanova, J. V. Sweedler, ACS Chem. Neurosci., 2018, 9, 1869 4. M. R. Bullock, B. G. Lyeth, J. P. Muizelaar, Neurosurgery, 1999, 45, 207 5. S. X. Li, B. W. Wang, D. Liu, G. L. He, H. Wang, Y. J. Duan, J. J. Xing, H. Y. Zhou, Y. W. Zhou, Fa Yi Xue Za Zhi, 2011, 27, 286, 294 6. L. D. Shultz, F. Ishikawa, D. L. Greiner, Nat. Rev. Immunol., 2007, 7, 118 7. D. E. Ingber, Nat. Rev. Genet., 2022, 23, 467 8. E. Suarez-Martinez, I. Suazo-Sanchez, M. Celis-Romero, A. Carnero, Cell Biosci., 2022, 12, 39 9. E. T. Verjans, J. Doijen, W. Luyten, B. Landuyt, L. Schoofs, J. Cell Physiol., 2018, 233, 2993 10. S. Kheiri, I. Yakavets, J. Cruickshank, F. Ahmadi, H. K. Berman, D. W. Cescon, E. W. K. Young, E. Kumacheva, Adv Mater., 2024, 36, 2410547 11. S. Ahadian, R. Civitarese, D. Bannerman, M. H. Mohammadi, R. Lu, E. Wang, L. Davenport-Huyer, B. Lai, B. Zhang, Y. Zhao, S. Mandla, A. Korolj, M. Radisic, Adv. Healthc. Mater., 2018, 7, 1700506 12. F. M. Pramotton, S. Spitz, R. D. Kamm, Adv. Sci. (Weinh), 2024, 11, e2403892 13. G. S. Offeddu, E. Cambria, S. E. Shelton, K. Haase, Z. Wan, L. Possenti, H. T. Nguyen, M. R. Gillrie, D. Hickman, C. G. Knutson, R. D. Kamm, Adv. Sci. (Weinh), 2024, 11, e2402757 14. A. R. Mulay, J. Hwang, D. H. Kim, Adv. Healthc. Mater., 2024, 13, e2303180 15. V. Carvalho, I. M. Gonçalves, N. Rodrigues, P. Sousa, V. Pinto, G. Minas, H. Kaji, S. R. Shin, R. O. Rodrigues, S. Teixeira, R. A. Lima, Comput. Methods Programs Biomed., 2024, 243, 107883 16. N. Tabatabaei Rezaei, H. Kumar, H. Liu, S. S. Lee, S. S. Park, K. Kim, Adv. Healthc. Mater., 2023, 12, e2203172 17. R. O. Rodrigues, S. R. Shin, M. Bañobre-López, J. Nanobiotechnology, 2024, 22, 573 18. Y. Hu, J. Xing, H. Zhang, X. Pang, Y. Zhai, H. Cheng, D. Xu, M. Liao, Y. Qi, D. Wu, B. Zhang, L. Cheng, B. Chu, C. Zhang, Y. Zhao, R. Chai, Adv. Mater., 2024, 36, e2309002 19. K. Ohashi, A. Hayashida, A. Nozawa, S. Ito, Curr. Res. Toxicol., 2024, 6, 100163 20. Z. Izadifar, J. Cotton, S. Chen, V. Horvath, A. Stejskalova, A. Gulati, N. T. LoGrande, B. Budnik, S. Shahriar, E. R. Doherty, Y. Xie, T. To, S. E. Gilpin, A. M. Sesay, G. Goyal, C. B. Lebrilla, D. E. Ingber, Nat. Commun., 2024, 15, 4578 21. K. E. de Roode, K. Hashemi, W. P. R. Verdurmen, R. Brock, Small, 2024, 20, e2402311 22. O. T. P. Nguyen, P. M. Misun, A. Hierlemann, C. Lohasz, Adv. Healthc. Mater., 2024, 13, e2302454 23. E. Jastrzebska, E. Tomecka, I. Jesion, Biosens. Bioelectron., 2016, 75, 67 24. I. Francis, J. Shrestha, K. R. Paudel, P. M. Hansbro, M. E. Warkiani, S. C. Saha, Drug Discov. Today, 2022, 27, 2593 25. K. T. Kroll, M. M. Mata, K. A. Homan, V. Micallef, A. Carpy, K. Hiratsuka, R. Morizane, A. Moisan, M. Gubler, A. C. Walz, E. Marrer-Berger, J. A. Lewis, Proc. Natl. Acad. Sci. USA, 2023, 120, e2305322120 26. Y. Hu, H. Zhang, S. Wang, L. Cao, F. Zhou, Y. Jing, J. Su, Bioact. Mater., 2023, 25, 29 27. T. Messelmani, L. Morisseau, Y. Sakai, C. Legallais, A. Le Goff, E. Leclerc, R. Jellali, Lab Chip, 2022, 22, 2423 28. E. Sutterby, P. Thurgood, S. Baratchi, K. Khoshmanesh, E. Pirogova, Small, 2020, 16, e2002515 29. G. D. Vatine, R. Barrile, M. J. Workman, S. Sances, B. K. Barriga, M. Rahnama, S. Barthakur, M. Kasendra, C. Lucchesi, J. Kerns, N. Wen, W. R. Spivia, Z. Chen, J. Van Eyk, C. N. Svendsen, Cell Stem Cell, 2019, 24, 995 30. K. Ronaldson-Bouchard, D. Teles, K. Yeager, D. N. Tavakol, Y. Zhao, A. Chramiec, S. Tagore, M. Summers, S. Stylianos, M. Tamargo, B. M. Lee, S. P. Halligan, E. H. Abaci, Z. Guo, J. Jacków, A. Pappalardo, J. Shih, R. K. Soni, S. Sonar, C. German, A. M. Christiano, A. Califano, K. K. Hirschi, C. S. Chen, A. Przekwas, G. Vunjak-Novakovic, Nat. Biomed. Eng., 2022, 6, 351 31. J. Ko, D. Park, S. Lee, B. Gumuscu, N. L. Jeon, Micromachines (Basel), 2022, 13, 1200 32. S. Breslin, L. O'Driscoll, Drug Discov. Today, 2013, 18, 240 33. K. Duval, H. Grover, L. H. Han, Y. Mou, A. F. Pegoraro, J. Fredberg, Z. Chen, Physiology (Bethesda), 2017, 32, 266 34. B. D. Cardoso, E. M. S. Castanheira, S. Lanceros-Méndez, V. F. Cardoso, Adv. Healthc. Mater., 2023, 12, e2202936 35. D. Lv, Z. Hu, L. Lu, H. Lu, X. Xu, Oncol Lett, 2017, 14, 6999 36. V. L. Dsouza, R. Kuthethur, S. P. Kabekkodu, S. Chakrabarty, Biochim. Biophys. Acta Rev. Cancer, 2022, 1877, 188717 37. W. H. Abuwatfa, W. G. Pitt, G. A. Husseini, J. Biomed. Sci., 2024, 31, 7 38. M. H. Kim, M. Kino-oka, M. Taya, Biotechnol. Adv., 2010, 28, 7 39. O. Habanjar, M. Diab-Assaf, F. Caldefie-Chezet, L. Delort, Int. J. Mol. Sci., 2021, 22, 12200 40. C. Vila-Parrondo, C. García-Astrain, L. M. Liz-Marzán, Adv. Colloid Interface Sci., 2020, 283, 102237 41. J. H. Ylostalo, Cells, 2020, 9, 2178 42. V. Brancato, J. M. Oliveira, V. M. Correlo, R. L. Reis, S. C. Kundu, Biomaterials, 2020, 232, 119744 43. S. Liu, S. Kumari, H. He, P. Mishra, B. N. Singh, D. Singh, S. Liu, P. Srivastava, C. Li, Biosens Bioelectron, 2023, 231, 115285 44. A. Zielińska, J. Karczewski, P. Eder, T. Kolanowski, M. Szalata, K. Wielgus, M. Szalata, D. Kim, S. R. Shin, R. Słomski, E. B. Souto, J Control Release, 2023, 359, 207 45. Y. Park, K. M. Huh, S. W. Kang, Int. J. Mol. Sci., 2021, 22, 2491 46. W. L. Stoppel, C. E. Ghezzi, S. L. McNamara, L. D. Black, 3rd, D. L. Kaplan, Ann. Biomed. Eng., 2015, 43, 657 47. P. B. Malafaya, G. A. Silva, R. L. Reis, Adv. Drug Deliv. Rev., 2007, 59, 207 48. S. Van Vlierberghe, P. Dubruel, E. Schacht, Biomacromolecules, 2011, 12, 1387 49. S. A. Yi, Y. Zhang, C. Rathnam, T. Pongkulapa, K. B. Lee, Adv. Mater., 2021, 33, e2007949 50. A. Abbott, Nature, 2003, 424, 870 51. T. Takahashi, Annu. Rev. Pharmacol. Toxicol., 2019, 59, 447 52. C. A. Trujillo, A. R. Muotri, Trends Mol. Med., 2018, 24, 982 53. C. S. Cowan, M. Renner, M. De Gennaro, B. Gross-Scherf, D. Goldblum, Y. Hou, M. Munz, T. M. Rodrigues, J. Krol, T. Szikra, R. Cuttat, A. Waldt, P. Papasaikas, R. Diggelmann, C. P. Patino-Alvarez, P. Galliker, S. E. Spirig, D. Pavlinic, N. Gerber-Hollbach, S. Schuierer, A. Srdanovic, M. Balogh, R. Panero, A. Kusnyerik, A. Szabo, M. B. Stadler, S. Orgül, S. Picelli, P. W. Hasler, A. Hierlemann, H. P. N. Scholl, G. Roma, F. Nigsch, B. Roska, Cell, 2020, 182, 1623 54. J. Lee, W. H. van der Valk, S. A. Serdy, C. Deakin, J. Kim, A. P. Le, K. R. Koehler, Nat. Protoc., 2022, 17, 1266 55. Y. R. Lewis-Israeli, A. H. Wasserman, M. A. Gabalski, B. D. Volmert, Y. Ming, K. A. Ball, W. Yang, J. Zou, G. Ni, N. Pajares, X. Chatzistavrou, W. Li, C. Zhou, A. Aguirre, Nat. Commun., 2021, 12, 5142 56. K. A. Homan, N. Gupta, K. T. Kroll, D. B. Kolesky, M. Skylar-Scott, T. Miyoshi, D. Mau, M. T. Valerius, T. Ferrante, J. V. Bonventre, J. A. Lewis, R. Morizane, Nat. Methods, 2019, 16, 255 57. S. J. Mun, J. S. Ryu, M. O. Lee, Y. S. Son, S. J. Oh, H. S. Cho, M. Y. Son, D. S. Kim, S. J. Kim, H. J. Yoo, H. J. Lee, J. Kim, C. R. Jung, K. S. Chung, M. J. Son, J. Hepatol., 2019, 71, 970 58. S. Rahmani, N. M. Breyner, H. M. Su, E. F. Verdu, T. F. Didar, Biomaterials, 2019, 194, 195 59. B. L. LeSavage, R. A. Suhar, N. Broguiere, M. P. Lutolf, S. C. Heilshorn, Nat. Mater., 2022, 21, 143 60. N. Werschler, C. Quintard, S. Nguyen, J. Penninger, Atherosclerosis, 2024, 398, 118529 61. S. C. Cordts, K. Yuki, M. F. Henao Echeverri, B. Narasimhan, C. J. Kuo, S. K. Y. Tang, Microsyst. Nanoeng., 2024, 10, 126 62. A. C. Ericsson, M. J. Crim, C. L. Franklin, Mo Med, 2013, 110, 201 63. K. R. Patil, U. B. Mahajan, B. S. Unger, S. N. Goyal, S. Belemkar, S. J. Surana, S. Ojha, C. R. Patil, Int. J. Mol. Sci., 2019, 20, 4376 64. E. E. Patton, L. I. Zon, D. M. Langenau, Nat. Rev. Drug Discov., 2021, 20, 611 65. U. B. Pandey, C. D. Nichols, Pharmacol. Rev., 2011, 63, 411 66. R. S. Prather, M. Lorson, J. W. Ross, J. J. Whyte, E. Walters, Annu. Rev. Anim. Biosci., 2013, 1, 203 67. K. A. Phillips, K. L. Bales, J. P. Capitanio, A. Conley, P. W. Czoty, B. A. t Hart, W. D. Hopkins, S. L. Hu, L. A. Miller, M. A. Nader, P. W. Nathanielsz, J. Rogers, C. A. Shively, M. L. Voytko, Am. J. Primatol., 2014, 76, 801 68. J. L. Rowell, D. O. McCarthy, C. E. Alvarez, Trends Mol. Med., 2011, 17, 380 69. G. L. Duff, M. G. Mc, A. C. Ritchie, Am J Pathol, 1957, 33, 845 70. J. Nourinezhad, V. Rostamizadeh, R. Ranjbar, Ann. Anat., 2022, 242, 151911 71. J. K. Lunney, A. Van Goor, K. E. Walker, T. Hailstock, J. Franklin, C. Dai, Sci. Transl. Med., 2021, 13, eabd5758 72. N. B. Robinson, K. Krieger, F. M. Khan, W. Huffman, M. Chang, A. Naik, R. Yongle, I. Hameed, K. Krieger, L. N. Girardi, M. Gaudino, Int. J. Surg., 2019, 72, 9 73. G. Qu, S. Wang, Z. Zhou, D. Jiang, A. Liao, J. Luo, Front. Immunol., 2022, 13, 891687 74. P. Pound, S. Ebrahim, P. Sandercock, M. B. Bracken, I. Roberts, Bmj, 2004, 328, 514 75. S. K. Doke, S. C. Dhawale, Saudi Pharm. J., 2015, 23, 223 76. Y. Zeng, Z. Gu, Chinese Science Bulletin, 2023, 68, 4954 77. D. Huh, G. A. Hamilton, D. E. Ingber, Trends Cell Biol., 2011, 21, 745 78. L. A. Low, C. Mummery, B. R. Berridge, C. P. Austin, D. A. Tagle, Nat. Rev. Drug Discov., 2021, 20, 345 79. J. Y. Shoji, R. P. Davis, C. L. Mummery, S. Krauss, Adv. Healthc. Mater., 2024, 13, e2301067 80. H. Liu, Y. Wang, K. Cui, Y. Guo, X. Zhang, J. Qin, Adv. Mater., 2019, 31, e1902042 81. G. Rossi, A. Manfrin, M. P. Lutolf, Nat. Rev. Genet., 2018, 19, 671 82. M. Chen, H. Shan, Q. Tao, R. Hu, Q. Sun, M. Zheng, Z. Chen, Q. Lin, M. Yin, S. Zhao, X. Chen, Z. Chen, Small, 2024, 20, e2308525 83. S. Wiedenmann, M. Breunig, J. Merkle, C. von Toerne, T. Georgiev, M. Moussus, L. Schulte, T. Seufferlein, M. Sterr, H. Lickert, S. E. Weissinger, P. Möller, S. M. Hauck, M. Hohwieler, A. Kleger, M. Meier, Nat. Biomed. Eng., 2021, 5, 897 84. T. Tao, P. Deng, Y. Wang, X. Zhang, Y. Guo, W. Chen, J. Qin, Adv. Sci. (Weinh), 2022, 9, e2103495 85. C. Quílez, E. Y. Jeon, A. Pappalardo, P. Pathak, H. E. Abaci, Adv. Healthc. Mater., 2024, 13, e2400405 86. S. E. Park, A. Georgescu, D. Huh, Science, 2019, 364, 960 87. V. S. Shirure, C. C. W. Hughes, S. C. George, Annu. Rev. Biomed. Eng., 2021, 23, 141 88. A. G. Monteduro, S. Rizzato, G. Caragnano, A. Trapani, G. Giannelli, G. Maruccio, Biosens. Bioelectron., 2023, 231, 115271 89. B. S. Elci, M. Nikolaev, S. Rezakhani, M. P. Lutolf, Adv. Healthc. Mater., 2024, 13, e2302912 90. O. Mitrofanova, M. Nikolaev, Q. Xu, N. Broguiere, I. Cubela, J. G. Camp, M. Bscheider, M. P. Lutolf, Cell Stem Cell, 2024, 31, 1175 91. N. Gjorevski, M. Nikolaev, T. E. Brown, O. Mitrofanova, N. Brandenberg, F. W. DelRio, F. M. Yavitt, P. Liberali, K. S. Anseth, M. P. Lutolf, Science, 2022, 375, eaaw9021 92. H. Jian, X. Li, Q. Dong, S. Tian, S. Bai, Cell Prolif., 2023, 56, e13465 93. V. Paloschi, J. Pauli, G. Winski, Z. Wu, Z. Li, L. Botti, S. Meucci, P. Conti, F. Rogowitz, N. Glukha, N. Hummel, A. Busch, E. Chernogubova, H. Jin, N. Sachs, H. H. Eckstein, A. Dueck, R. A. Boon, A. R. Bausch, L. Maegdefessel, Adv. Healthc. Mater., 2024, 13, e2302907 94. Y. Jun, J. Lee, S. Choi, J. H. Yang, M. Sander, S. Chung, S. H. Lee, Sci. Adv., 2019, 5, eaax4520 95. B. Waclawiková, A. Codutti, K. Alim, S. El Aidy, Gut Microbes, 2022, 14, 1997296 96. A. Grassart, V. Malardé, S. Gobaa, A. Sartori-Rupp, J. Kerns, K. Karalis, B. Marteyn, P. Sansonetti, N. Sauvonnet, Cell Host Microbe, 2019, 26, 435 97. K. H. Vining, D. J. Mooney, Nat. Rev. Mol. Cell Biol., 2017, 18, 728 98. T. Mammoto, D. E. Ingber, Development, 2010, 137, 1407 99. M. Niu, Y. Zhu, X. Ding, Y. Zu, Y. Zhao, Y. Wang, Adv. Healthc. Mater., 2023, 12, e2300850 100. D. Xu, Y. Wang, L. Sun, Z. Luo, Y. Luo, Y. Wang, Y. Zhao, ACS Nano, 2023, 17, 15180 101. Y. Zhu, L. Sun, Y. Wang, L. Cai, Z. Zhang, Y. Shang, Y. Zhao, Adv. Mater., 2022, 34, e2108972 102. Y. Zhang, Y. Wang, H. Yin, J. Wang, N. Liu, S. Zhong, L. Li, Q. Zhang, T. Yue, Microsyst. Nanoeng., 2024, 10, 88 103. K. Achberger, C. Probst, J. Haderspeck, S. Bolz, J. Rogal, J. Chuchuy, M. Nikolova, V. Cora, L. Antkowiak, W. Haq, N. Shen, K. Schenke-Layland, M. Ueffing, S. Liebau, P. Loskill, elife, 2019, 8, e46188 104. T. A. V. Afanasyeva, J. C. Corral-Serrano, A. Garanto, R. Roepman, M. E. Cheetham, R. W. J. Collin, Cell Mol. Life Sci., 2021, 78, 6505 105. E. Garreta, R. D. Kamm, S. M. Chuva de Sousa Lopes, M. A. Lancaster, R. Weiss, X. Trepat, I. Hyun, N. Montserrat, Nat. Mater., 2021, 20, 145 106. R. Zandi Shafagh, S. Youhanna, J. Keulen, J. X. Shen, N. Taebnia, L. C. Preiss, K. Klein, F. A. Büttner, M. Bergqvist, W. van der Wijngaart, V. M. Lauschke, Adv. Sci. (Weinh), 2022, 9, e2203368 107. V. V. T. Nguyen, S. Ye, V. Gkouzioti, M. E. van Wolferen, F. Y. Yengej, D. Melkert, S. Siti, B. de Jong, P. J. Besseling, B. Spee, L. J. W. van der Laan, R. Horland, M. C. Verhaar, B. W. M. van Balkom, J. Extracell. Vesicles, 2022, 11, e12280 108. S. Bauer, C. Wennberg Huldt, K. P. Kanebratt, I. Durieux, D. Gunne, S. Andersson, L. Ewart, W. G. Haynes, I. Maschmeyer, A. Winter, C. Ämmälä, U. Marx, T. B. Andersson, Sci. Rep., 2017, 7, 14620 109. D. Bovard, A. Sandoz, K. Luettich, S. Frentzel, A. Iskandar, D. Marescotti, K. Trivedi, E. Guedj, Q. Dutertre, M. C. Peitsch, J. Hoeng, Lab Chip, 2018, 18, 3814 110. J. W. Jeon, S. H. Lee, D. Kim, J. H. Sung, Biotechnol. Prog., 2021, 37, e3121 111. D. N. Tavakol, T. R. Nash, Y. Kim, P. L. Graney, M. Liberman, S. Fleischer, R. I. Lock, A. O'Donnell, L. Andrews, D. Ning, K. Yeager, A. Harken, N. Deoli, S. A. Amundson, G. Garty, K. W. Leong, D. J. Brenner, G. Vunjak-Novakovic, Adv. Sci. (Weinh), 2024, 11, e2401415 112. R. Janssen, J. W. M. de Kleer, B. Heming, S. Bastiaan-Net, J. Garssen, L. E. M. Willemsen, R. Masereeuw, Trends Biotechnol., 2024, 42, 119 113. J. H. Sung, Y. I. Wang, N. Narasimhan Sriram, M. Jackson, C. Long, J. J. Hickman, M. L. Shuler, Anal. Chem., 2019, 91, 330 114. M. B. Esch, G. J. Mahler, T. Stokol, M. L. Shuler, Lab Chip, 2014, 14, 3081 115. Y. Suhail, M. P. Cain, K. Vanaja, P. A. Kurywchak, A. Levchenko, R. Kalluri, Kshitiz, Cell Syst., 2019, 9, 109 116. N. Del Piccolo, V. S. Shirure, Y. Bi, S. P. Goedegebuure, S. Gholami, C. C. W. Hughes, R. C. Fields, S. C. George, Adv. Drug Deliv. Rev., 2021, 175, 113798 117. D. Caballero, S. Kaushik, V. M. Correlo, J. M. Oliveira, R. L. Reis, S. C. Kundu, Biomaterials, 2017, 149, 98 118. Z. Zou, Z. Lin, C. Wu, J. Tan, J. Zhang, Y. Peng, K. Zhang, J. Li, M. Wu, Y. Zhang, Adv. Sci. (Weinh), 2023, 10, e2302640 119. C. Ma, Y. Peng, H. Li, W. Chen, Trends Pharmacol. Sci., 2021, 42, 119 120. J. Huang, Z. Xu, J. Jiao, Z. Li, S. Li, Y. Liu, Z. Li, G. Qu, J. Wu, Y. Zhao, K. Chen, J. Li, Y. Pan, X. Wu, J. Ren, Bioact. Mater., 2023, 30, 1 121. Y. Zhu, D. Jiang, Y. Qiu, X. Liu, Y. Bian, S. Tian, X. Wang, K. J. Hsia, H. Wan, L. Zhuang, P. Wang, Bioact. Mater., 2024, 39, 59 122. M. Zhang, P. Wang, R. Luo, Y. Wang, Z. Li, Y. Guo, Y. Yao, M. Li, T. Tao, W. Chen, J. Han, H. Liu, K. Cui, X. Zhang, Y. Zheng, J. Qin, Adv. Sci. (Weinh), 2021, 8, 2002928 123. V. Negi, D. Gavlock, M. T. Miedel, J. K. Lee, T. Shun, A. Gough, L. Vernetti, A. M. Stern, D. L. Taylor, V. K. Yechoor, Lab Chip, 2023, 23, 4514 124. D. Butler, D. R. Reyes, Lab Chip, 2024, 24, 1494 125. S. Park, T. C. Laskow, J. Chen, P. Guha, B. Dawn, D. H. Kim, Aging Cell, 2024, 23, e14070 126. W. Balestri, R. Sharma, V. A. da Silva, B. C. Bobotis, A. J. Curle, V. Kothakota, F. Kalantarnia, M. V. Hangad, M. Hoorfar, J. L. Jones, M. Tremblay, J. J. El-Jawhari, S. M. Willerth, Y. Reinwald, J Neuroinflammation, 2024, 21, 32 127. J. Ko, J. Song, Y. Lee, N. Choi, H. N. Kim, Lab Chip, 2024, 24, 1542 128. B. J. Kwee, X. Li, X. X. Nguyen, C. Campagna, J. Lam, K. E. Sung, Exp. Biol. Med. (Maywood), 2023, 248, 2001 129. K. Ge, Y. Ren, Z. Hong, Z. Mao, B. Yao, K. Ye and C. Jia, Adv. Healthc. Mater., 2024, 13, e2401990 130. L. H. Goetz, N. J. Schork, Fertil. Steril., 2018, 109, 952 131. P. C. Beltrão-Braga, G. C. Pignatari, F. B. Russo, I. R. Fernandes, A. R. Muotri, Cytometry A, 2013, 83, 11 132. Y. A. Jodat, M. G. Kang, K. Kiaee, G. J. Kim, A. F. H. Martinez, A. Rosenkranz, H. Bae, S. R. Shin, Curr Pharm Des, 2018, 24, 5471 133. E. W. Esch, A. Bahinski, D. Huh, Nat. Rev. Drug Discov., 2015, 14, 248 134. U.S. Food and Drug Administration (FDA), FDA Voice: Organs-on-Chips Technology, https://wayback.archive-it.org/8521/20180903192020/https://blogs.fda.gov/fdavoice/index.php/tag/organs-on-chips-technology/, September 2018. 135. U.S. Food and Drug Administration (FDA), Advancing New Alternative Methodologies at FDA, https://www.fda.gov/media/144891/download, January 2021. 136. Valuates Reports, Global Organs-on-Chips (OOC) Market Report, https://reports.valuates.com/market-reports/QYRE-Auto-9N6280/global-organs-on-chips-ooc, 2024. 137. S. Deng, C. Li, J. Cao, Z. Cui, J. Du, Z. Fu, H. Yang, P. Chen, Theranostics, 2023, 13, 4526 138. M. Busek, S. Nøvik, A. Aizenshtadt, M. Amirola-Martinez, T. Combriat, S. Grünzner, S. Krauss, Biosensors (Basel), 2021, 11, 162 139. M. X. Doss, A. Sachinidis, Cells, 2019, 8, 403 140. T. Ching, Y. C. Toh, M. Hashimoto, Y. S. Zhang, Trends Pharmacol. Sci., 2021, 42, 715 -
Rights and permissions
This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.
Information
Article Metrics
-
Figure 1.
The schematic diagram of organ-on-chips. Various OOCs for the human body are introduced in the outer circle, three applications of OOC are introduced on the left side of the inner circle, and three ways of OOC fabrication are introduced on the right side. Source: Created with BioRender.com.
-
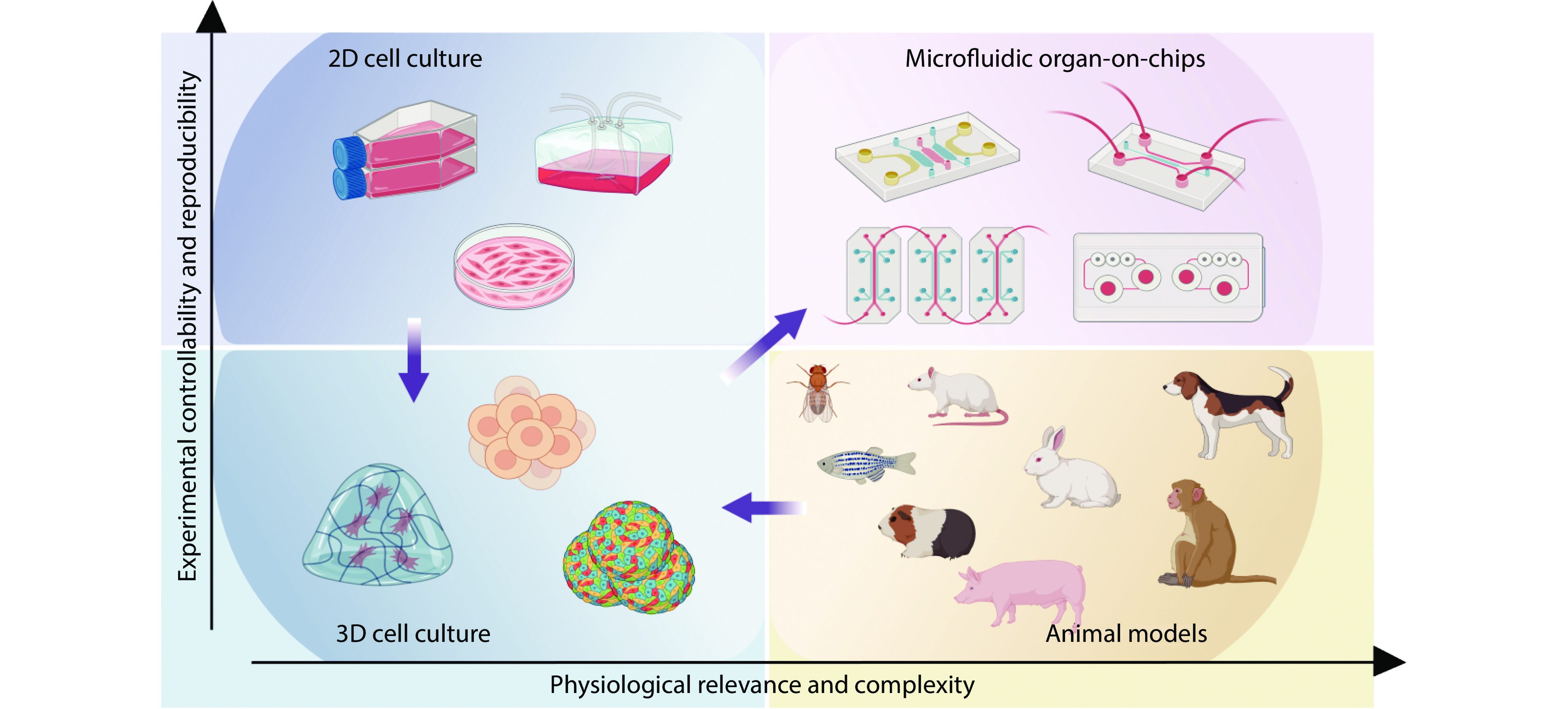
Figure 2.
This picture introduces four tools for preclinical drug screening and disease modeling: 2D cell culture, 3D cell culture and organ-on-chip models in vitro, and small and large animal models such as mice and pigs in vivo. Source: Created with BioRender.com.
-
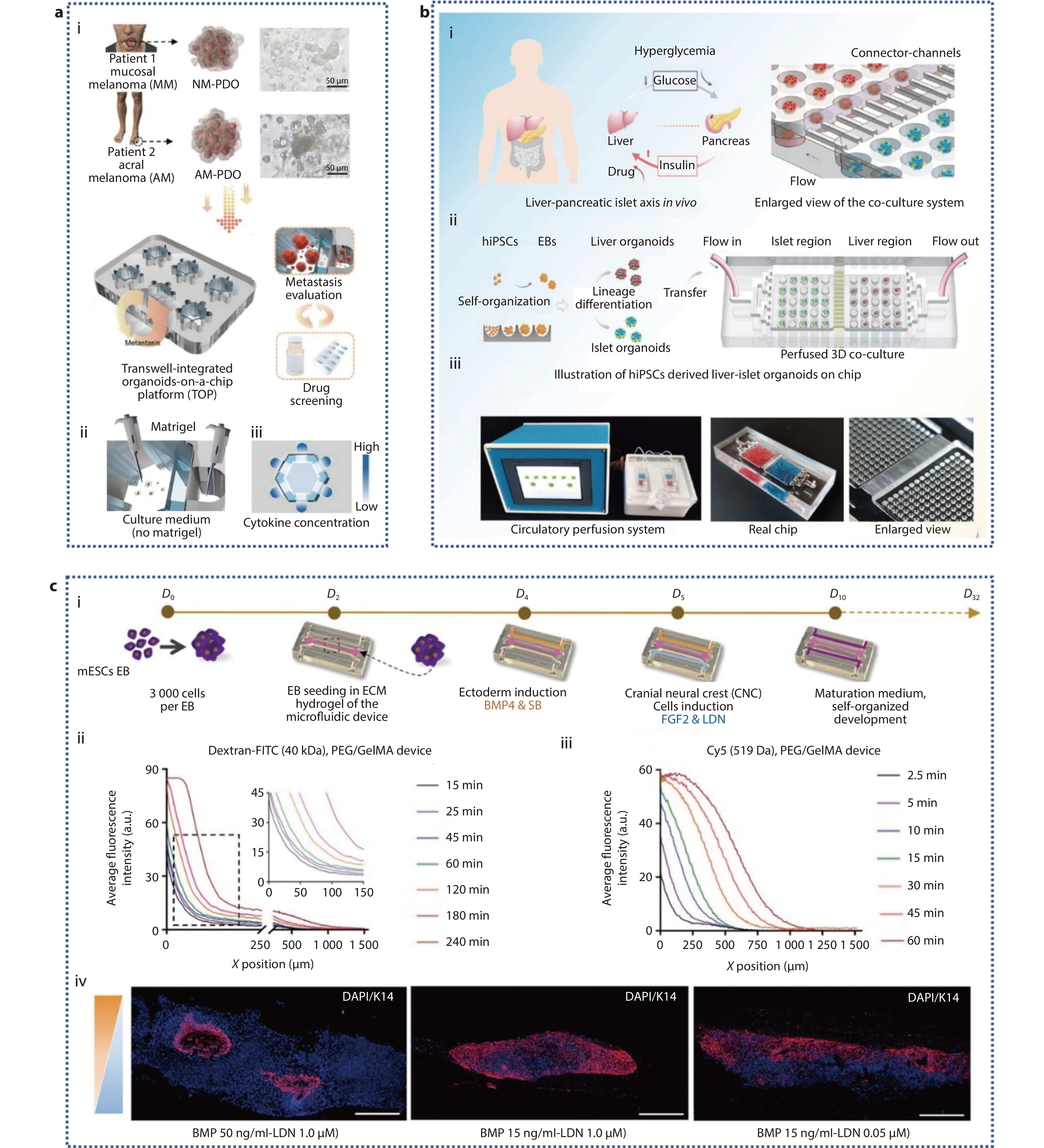
Figure 3.
a (i) Schematic diagram of the TOP system's capabilities; (ii) Schematic of the distribution of Matrigel in the TOP; (iii) Schematic illustration of the cytokine concentration gradient in the TOP.[82] Copyright 2024, Wiley-VCH GmbH. b Schematic of hiPSCs derived multi-organoid-on-chip system in vitro. (i) Illustration of how human liver and pancreatic islet tissues regulate glucose levels in vivo; (ii) The differentiation, formation, and co-culture process of liver and pancreatic islet organoids derived from hiPSCs in the microwell device; (iii) Microfluidic perfusion system device and chip physical diagram.[84] Copyright 2021, The Authors. Advanced Science published by Wiley-VCH GmbH. c (i) Schematic representation of PSO culture within a microfluidic system; (ii) Analysis of the chemical gradient for molecules of A) high (40 kDa) and B) low (519 Da) molecular weight; (iv) IF images of Krt14 (in red) in PSO cultures under different conditions on day 32 within the device.[85] Copyright 2024, Wiley-VCH GmbH.
-
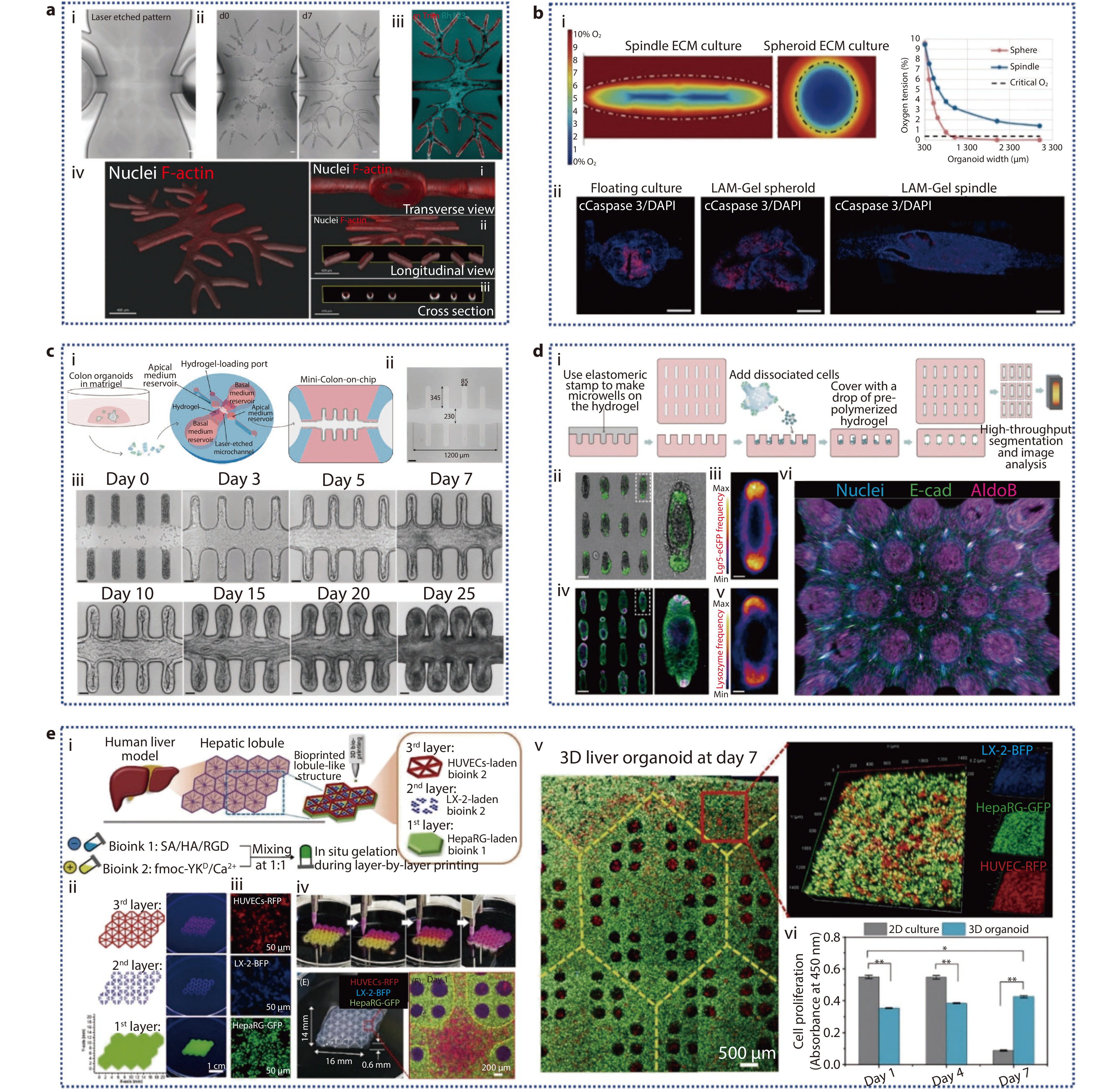
Figure 4.
a The formation of the complex biliary tree within prepatterned hydrogel chips. (i) Laser-ablated channel diameter of the branch network is from 150 to 50 μm; (ii) Time-lapse bright field images depicting the epithelium formation following repopulating by organoid-derived cholangiocytes from d0 (left) to d7 (right); (iii) Live-imaging of Rhodamine 123 in the structure at 10 days from delivery; (iv) 3D reconstruction of the entire construct, along with three distinct views on day 8, showing Nuclei and F-actin staining.[89] Copyright 2024, Wiley-VCH GmbH. b (i) Numerical simulation comparing the O2 concentration profiles within PSOs grown in 3D ECM as a sphere versus as a spindle shape in the spindle device; (ii) On day 32, IF images of Caspase 3 and DAPI in PSO.[85] Copyright 2024, Wiley-VCH GmbH. c (i) Schematic representation of the workflow for generating human mini-colons; (ii) Laser ablation creates an open microchannel with specific dimensions; (iii) Bright-field time-course imaging of the formation of epithelium in mini-colons.[90] Copyright 2024, The Author(s). Published by Elsevier Inc. d (i) The generation process of microfabricated tissues; (ii) A collection of intestinal organoids derived from engineered intestinal tissues with a rod-like shape and specified magnification; (iii) Frequency map of Lgr 5, showing average Lgr 5 expression over around 80 tissues; (iv) Lysozyme staining of Paneth cells in the array of intestinal organoids; v) average Paneth cell distribution; (vi) 3D reconstruction of the immunofluorescence images.[91] Copyright 2022, The American Association for the Advancement of Science. e (i) Illustration of the in vitro fabrication of liver organoids featuring biomimetic lobular architecture through a multi-cellular 3D bioprinting approach; (ii) Programming of 3D droplet-based bioprinting for the in vitro liver model fabrication; (iii) Multicellular composition of the assembled liver organoid; (iv) Printing process photos; 3D liver organoid and its hydrogel scaffold optical image; confocal microscopy image showing the hexagonal central region within the lobule-like structure following 1 day of culture; (v) Confocal images of the assembled liver organoids following 7 days of culture; (vi) Cell proliferation assay results.[92] Copyright 2023, The Authors. Cell Proliferation published by Beijing Institute for Stem Cell and Regenerative Medicine and John Wiley & Sons Ltd.
-
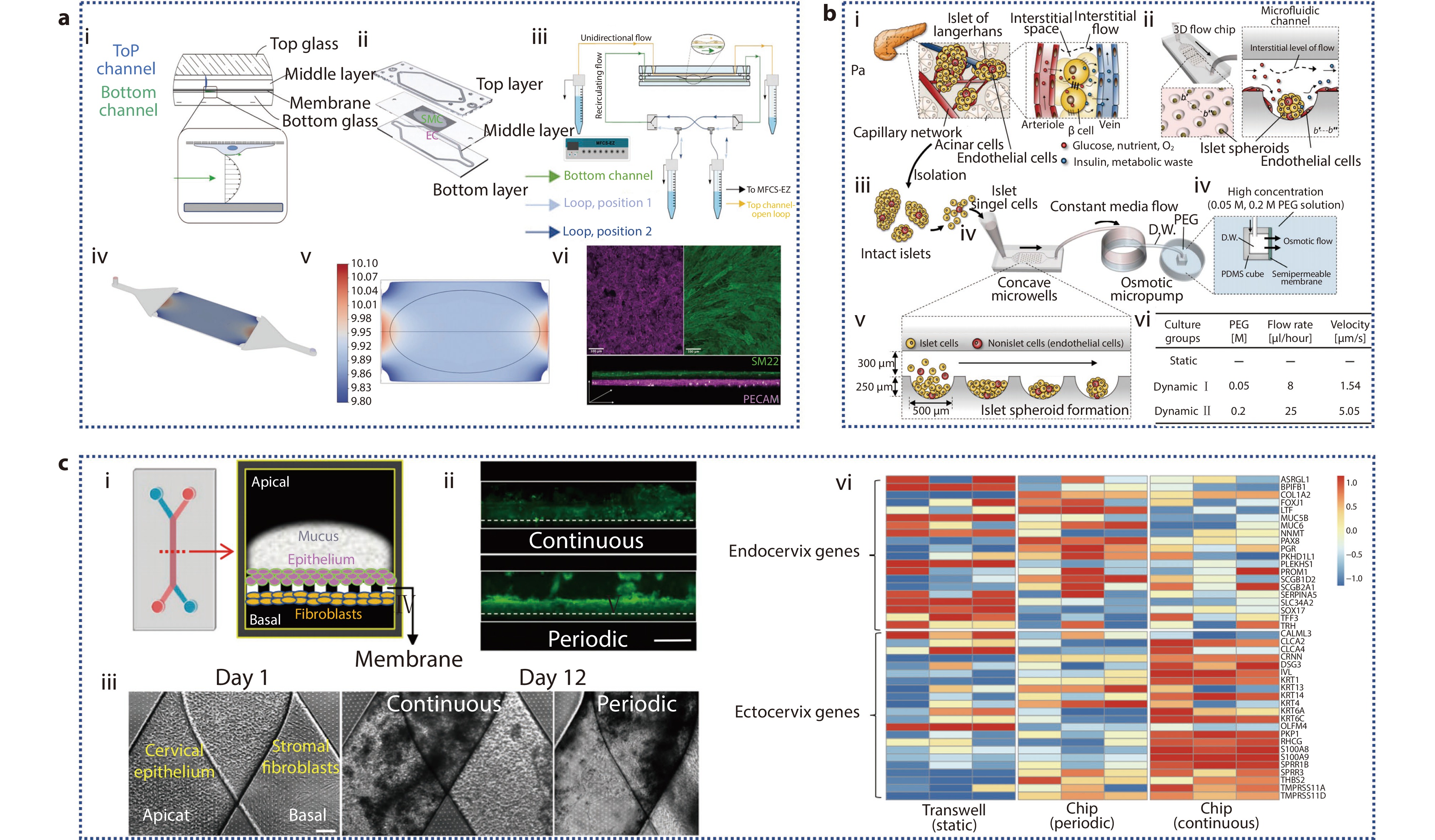
Figure 5.
a (i) Cross-sectional design of AoC model; (ii) EC, SMC co-culture membrane schematic diagram; (iii) Schematic diagram of AoC and microfluidic pump; (iv) 3D view of the bottom channel; (v) 2D diagram of wall shear stress distribution along film; (vi) Immunofluorescence staining of EC and SMC on the membrane.[93] Copyright 2023, The Authors. Advanced Healthcare Materials published by Wiley-VCH GmbH. b Spheroid-based microfluidic perfusion culture of pancreatic islets used to simulate the in vivo environment. (i) Clusters of endocrine cells are scattered throughout the exocrine acini in the native pancreas, forming islets of Langerhans; (ii) Engineering of islet spheroids on a microchip with a perfusion system is implemented to simulate in vivo environment; (iii-v) Schematics of the experimental setup; (vi) Pancreatic islet cells cultured in microfluidic chips under three disparate environments.[94] Copyright 2019, The American Association for the Advancement of Science. c Progression and characterization of human ecto-and endo-cervix chips. (i) Schematic diagram and cross-sectional design of two-channel microfluidic organ chip; (ii) A fluorescent microscopy side view shows the mucous layer in live Cervix Chip cultures, stained with wheat germ agglutinin (WGA) fluorescence (green), after seven days of differentiation. The images compare the effects of continuous flow (top) and periodic flow (bottom) regimens (bar, 1 mm); (iii) Phase-contrast microscopy top view of a Cervix Chip, with cervical epithelium on the apical side and fibroblasts on the basal side of the porous membrane, captured on day 1 (left) and after 12 days of culture under continuous (middle) or periodic (right) flow conditions (bar, 200 μm); (iv) Cervical epithelial cells’ gene expression from three different donors were RNAseq analyzed and differentiated into static Transwell or Cervix chips in either a periodic or continuous flow.[20] Copyright 2024, The Author(s).
-
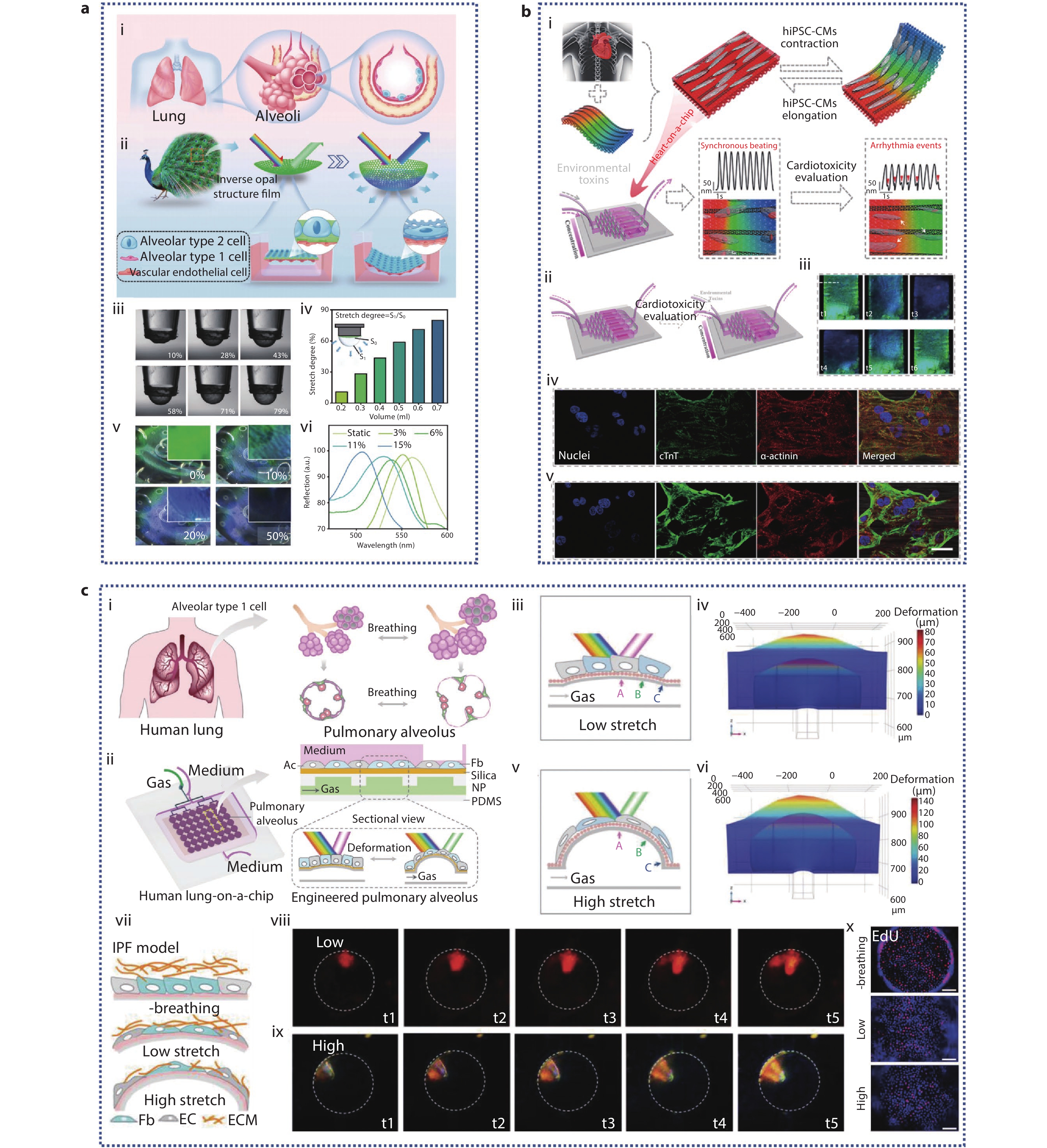
Figure 6.
a (i-ii) Schematic illustration of a simulated alveolar device with IOS-PU film; (iii-vi) Characterization of alveolar-mimicking devices; (iii-iv) The relationship between tensile degree and perfusion volume; (v) The relationship between tensile degree and film color; (vi) Changes of reflection spectra of IOS-PU films under different stretching degree.[99] Copyright 2023, Wiley-VCH GmbH. b (i) Schematic representation of the living anisotropic structural color hydrogels used for screening cardiotoxicity caused by environmental toxins; (ii) Heart-on-a-chip platform; (iii) Optical microscopic images showing structural color variations throughout a beating cycle of hiPSC-derived cardiomyocytes (hiPSC-CMs); (iv-v) The CLSM images of hiPSC-CMs treated with 75 μg/mL PQ (iv) and 25 μM CdCl2 (v) were sustained for 24 hours when anisotropic structural color hydrogels in vivo were evaluated for environmental toxins.[100] Copyright 2023, American Chemical Society. c (i-ii) Principle and schematic diagram of bionic human lung chip with microphysiological respiration visualization; (iii-vi) Engineered pulmonary alveolus deform under low and high cycle stretching; Low (iii-iv) and high (v-vi) flow PDMS film deformation diagram and numerical simulation of film deformation at peak cycle; (vii) Schematic of idiopathic pulmonary fibrosis (IPF) model by exposure to TGF-β; (viii-ix) Optical microscope images of structural color changes of low and high airflow during breathing under IPF model; (x) EdU staining (red) of co-cultured HFL1 and HPAEpiC cells, with or without cyclic stretching after TGF-ββ treatment.[101] Copyright 2022, Wiley-VCH GmbH.
-
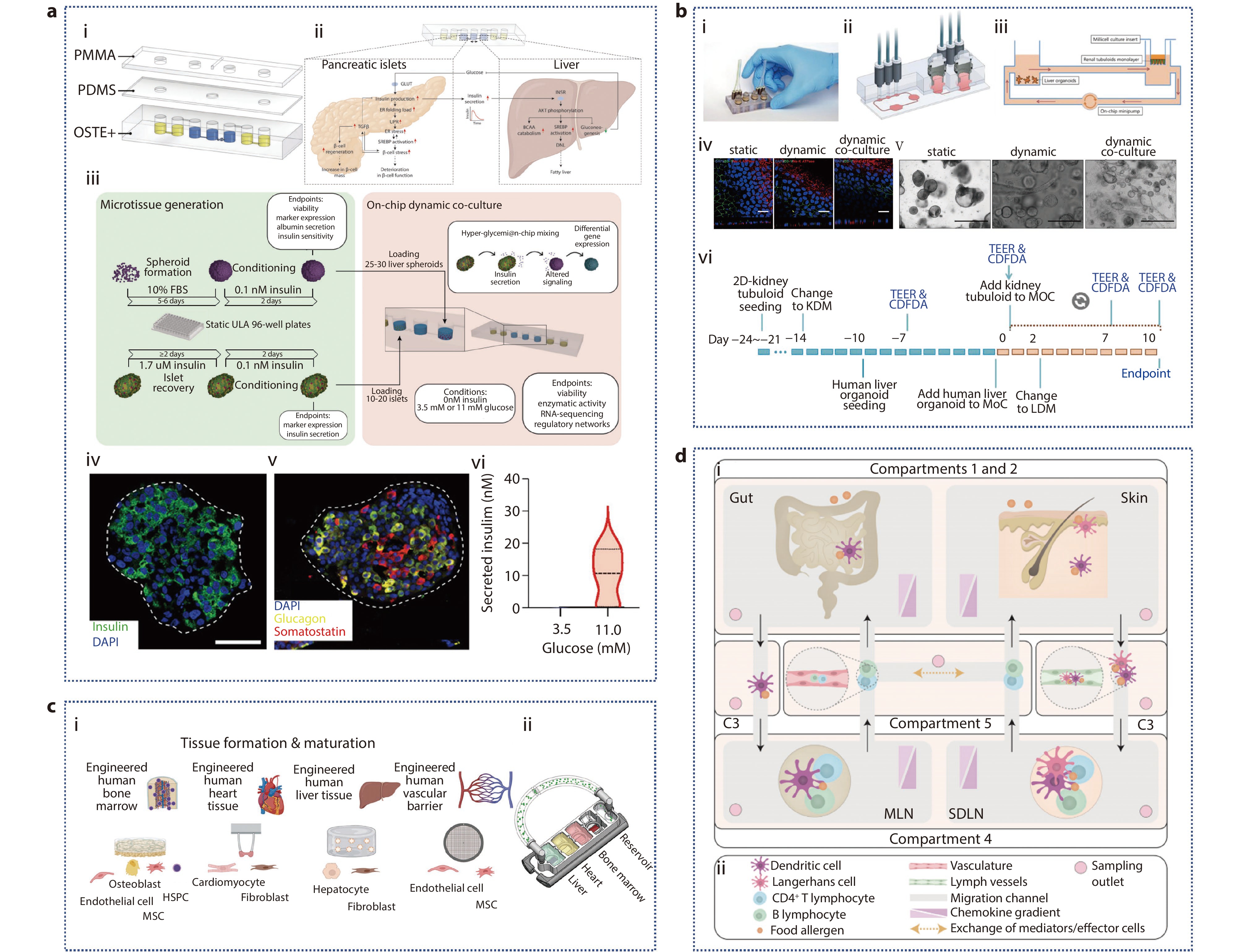
Figure 7.
a (i) Pneumatic hybrid microfluidic device; (ii) Schematic diagram of molecular networks interacting in prediabetic hyperglycemia; (iii) Pancreatic islets and liver spheroids are generated and recovered using ultralow attachment (ULA) plates; (iv-v) Immunofluorescent staining of primary human islets for insulin (β-cells), glucagon (α-cells), and somatostatin (δ-cells); (vi) Quantification of insulin secretion following exposure of islets to hypoglycemic or hyperglycemic conditions.[106] Copyright 2022, The Authors. Advanced Science published by Wiley-VCH GmbH. b (i-iii) Characterization of renal tubule. (i) photographs; (ii) cross-sectional view; (iii) schematic illustration; (iv) Immunofluorescence staining of 2D-culture renal tubuloids in static, dynamic, and dynamic co-culture with DAPI-stained nuclei (blue), the tight junction protein ZO-1 (green), and the Na/K-ATPase (red) showing polarized epithelial cell layers in all conditions; (v) Static, dynamic, and dynamic co-culture conditions of liver organoids; (vi) Experimental timeline. KDM: kidney differentiation medium, LDM: liver differentiation medium.[107] Copyright 2022, The Authors. Journal of Extracellular Vesicles published by Wiley Periodicals, LLC on behalf of the International Society for Extracellular Vesicles. c Multi-organ chip of bone marrow-heart-liver-vascular.[111] Copyright 2024, The Author(s). Advanced Science published by Wiley-VCH GmbH. d (i) A compartmentalized and functional gut-immune-skin OOC device; (ii) Legend for (i).[112] Copyright 2023, Author(s). Published by Elsevier Ltd.
-
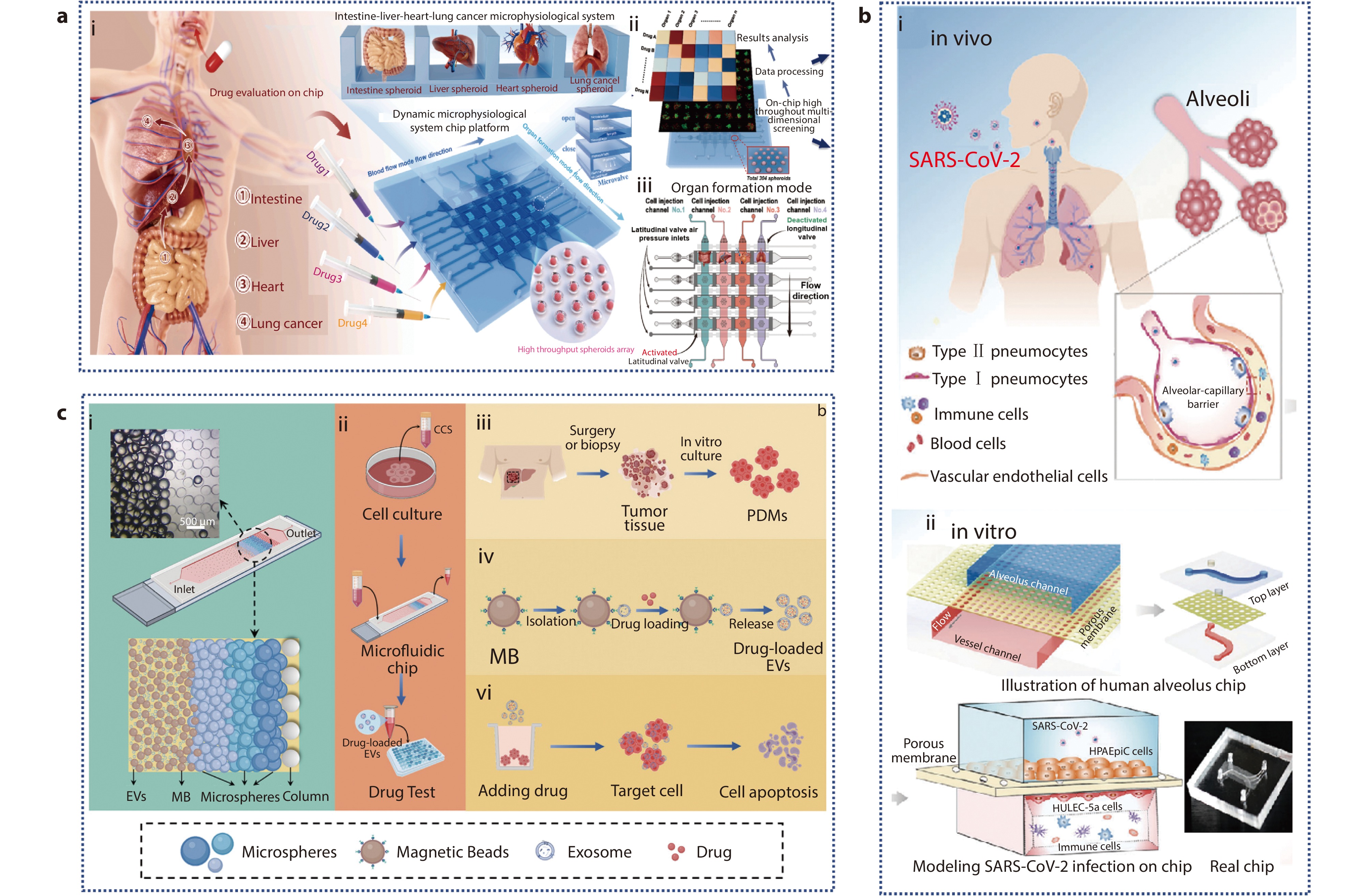
Figure 8.
a (i) Schematic of the absorption process of oral anti-lung cancer drugs and construction of a high-throughput intestine-liver-heart-lung cancer microphysiological system, which can evaluate four different drugs at the same time; (ii) Application of MSCP platform; (iii) The schematic representation of the organ formation mode.[121] Copyright 2024, The Authors. Publishing services by Elsevier B.V. on behalf of KeAi Communications Co. Ltd. b (i) 3D human alveolar-capillary barrier model in vivo; (ii) Configuration of a bionic human alveolus chip that is infected by SARS-CoV-2.[122] Copyright 2020, The Authors. Advanced Science published by Wiley-VCH GmbH. c (i) Schematic diagram of microfluidic chip architecture; (ii-vi) Application of PT-EVs in homologous tumor therapy.[129] Copyright 2024, Wiley-VCH GmbH.

 Xinyue Han graduated from the Northeast Agricultural University in 2023 with a bachelor's degree. She is currently studying for her doctorate in the School of Medicine of Southeast University. Her current research interests focus on organoid and organ-on-a-chip technology.
Xinyue Han graduated from the Northeast Agricultural University in 2023 with a bachelor's degree. She is currently studying for her doctorate in the School of Medicine of Southeast University. Her current research interests focus on organoid and organ-on-a-chip technology.  Yuyang Qiu graduated from the School of Life Sciences of Anhui University in 2023 with a bachelor's degree. She is currently studying for her doctorate in the School of Medicine of Southeast University. Her current research interests focus on inner ear drug delivery and tissue engineering.
Yuyang Qiu graduated from the School of Life Sciences of Anhui University in 2023 with a bachelor's degree. She is currently studying for her doctorate in the School of Medicine of Southeast University. Her current research interests focus on inner ear drug delivery and tissue engineering.  Bin Zhang graduated with a Master's degree in Jiangsu Key Laboratory of Neuroregeneration at Nantong University in 2021. She is currently pursuing her doctoral studies at the School of Public Health at Southeast University. Her current research interests are centered on nerve repair, organ-on-chip technology, and the development of biomaterials for inner ear drug delivery, along with their potential applications in the field.
Bin Zhang graduated with a Master's degree in Jiangsu Key Laboratory of Neuroregeneration at Nantong University in 2021. She is currently pursuing her doctoral studies at the School of Public Health at Southeast University. Her current research interests are centered on nerve repair, organ-on-chip technology, and the development of biomaterials for inner ear drug delivery, along with their potential applications in the field.  Yunzhu Huang is a lab assistant at Southeast University. She holds a Bachelor’s degree in Chemistry with a minor in Mathematics from Colby College (2024). Her research interests include material science.
Yunzhu Huang is a lab assistant at Southeast University. She holds a Bachelor’s degree in Chemistry with a minor in Mathematics from Colby College (2024). Her research interests include material science.  Qian Zhu received her Ph.D. degree in Academy of Military Science, and now worked as an associate researcher at Zhongda Hospital. Her current scientific interest focuses on Organoids-on-Chips and neural regeneration.
Qian Zhu received her Ph.D. degree in Academy of Military Science, and now worked as an associate researcher at Zhongda Hospital. Her current scientific interest focuses on Organoids-on-Chips and neural regeneration.  Menghui Liao received her Ph.D. from the School of Life Science and Technology at Southeast University in 2023. She is currently a postdoctoral researcher at the same institution under the supervision of Professor Renjie Chai. Her research primarily focuses on biomedical materials and auditory function restoration.
Menghui Liao received her Ph.D. from the School of Life Science and Technology at Southeast University in 2023. She is currently a postdoctoral researcher at the same institution under the supervision of Professor Renjie Chai. Her research primarily focuses on biomedical materials and auditory function restoration.  Yangnan Hu received her doctoral degree from the School of Life Science and Technology, Southeast University in 2023. Now, she is working as a postdoctoral fellow at the School of Life Science and Technology of Southeast University under the supervision of Prof. Renjie Chai. Her current scientific interest is focused on inner ear organoids and tissue engineering.
Yangnan Hu received her doctoral degree from the School of Life Science and Technology, Southeast University in 2023. Now, she is working as a postdoctoral fellow at the School of Life Science and Technology of Southeast University under the supervision of Prof. Renjie Chai. Her current scientific interest is focused on inner ear organoids and tissue engineering.  Renjie Chai is a chief professor at Southeast University. He received his Ph.D. degree from Baylor University in 2009 and worked as a postdoctoral fellow at Stanford University from 2009 to 2013. He has long been committed to the regeneration and protection of inner ear hair cells and spiral ganglion neurons, as well as the transcriptional regulation mechanism of neural stem cells and inner ear stem cells.
Renjie Chai is a chief professor at Southeast University. He received his Ph.D. degree from Baylor University in 2009 and worked as a postdoctoral fellow at Stanford University from 2009 to 2013. He has long been committed to the regeneration and protection of inner ear hair cells and spiral ganglion neurons, as well as the transcriptional regulation mechanism of neural stem cells and inner ear stem cells. 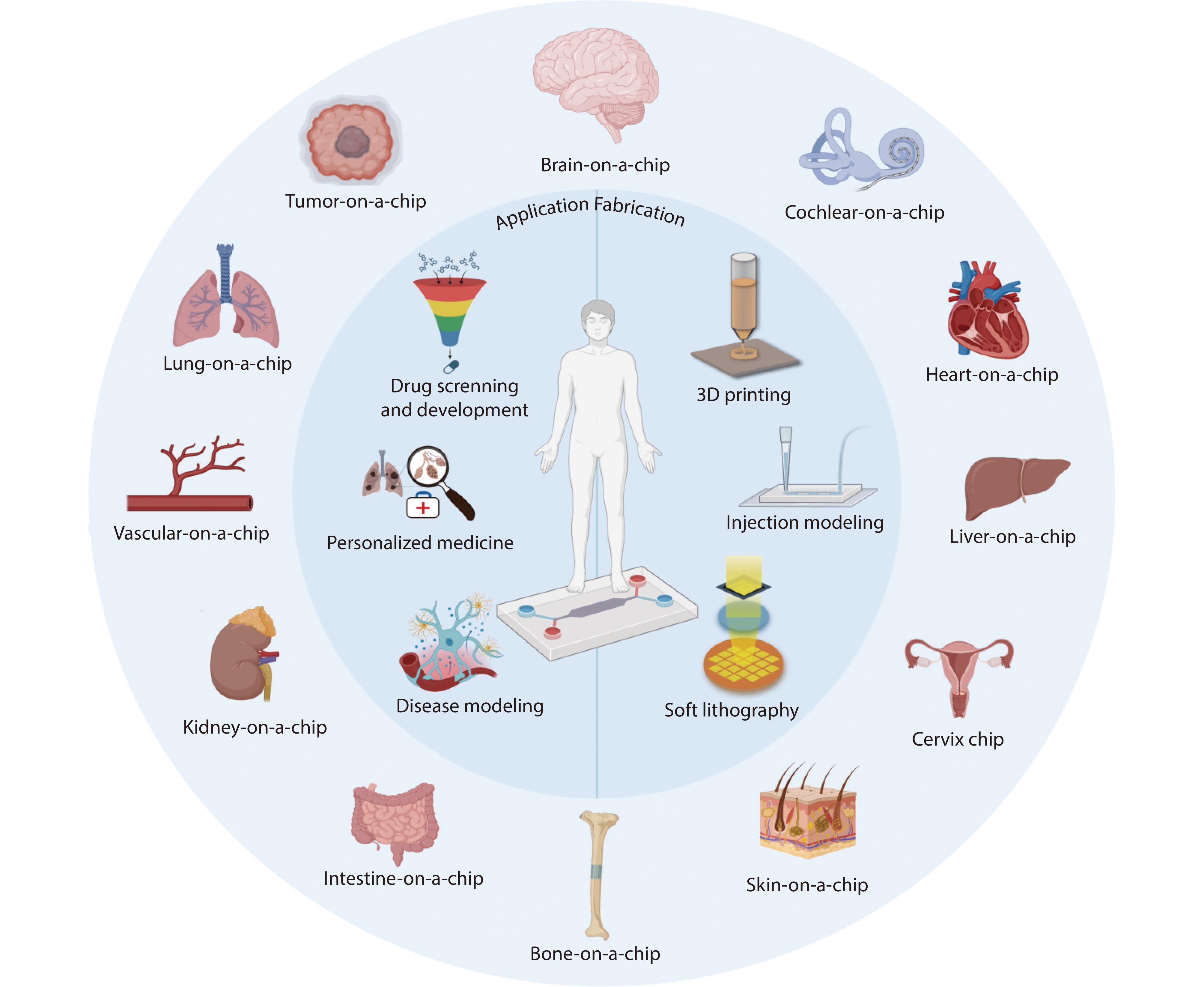

 DownLoad:
DownLoad: